H2 Molecular Orbital Diagram
A bonding mo and an anti bonding mo. Molecular orbital diagrams of diatomic molecules introduction.
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
This mo has an increased probability of finding electrons in the bonding region.

H2 molecular orbital diagram. Molecular orbitals of h 2 and he2. Many theories have been developed using them as models. This results in a bonding sigma mo σ 1s.
Bonding and anti bonding molecular orbitals in h 2. Dashed lines connect the parent atomic orbitals with the daughter molecular orbitals. A diatomic molecular orbital diagram is used to understand the bonding of a diatomic molecule.
Two superpositions of these two orbitals can be formed one by summing the orbitals and the other by taking their difference. Each h atom has a 1s atomic orbital. Molecular orbital mo theory of the h2 molecule.
πε and jr k r mr lr defined explicitly in atkins. Bonding mos antibonding mos and bond order. If the electrons are in phase they have a constructive interference.
In the middle of the diagram the molecular orbitals of the molecule of interest are written. Ab a b gg with. Mo diagrams can be used to deduce magnetic properties of a molecule and how they change with ionization.
The hydrogen atom is the simplest atom and its molecule ceh2 is also the simplest molecule than monoatomic molecules such as cehe and cene etc. Molecular orbitals of h 2. In order to predict the bond order molecular orbital diagram for h2 is to be drawn.
Evaluate the ground state electronic energy based on this presumed approximate eigenfunction. Because of their simplicity they have been extensively studied. In chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule.
When two h atoms come to a proper proximity their 1s orbitals interact and produce two molecular orbitals. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms. Its molecular orbitals are constructed from the valence shell orbitals of each hydrogen atom which are the 1 s orbitals of the atoms.
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. In general bonding molecular orbitals are lower in energy than either of their parent atomic orbitals. When two hydrogen atoms come closer then on combining two 1s orbitalstwo molecular orbitals are formed among which one is bonding and other is antibonding molecular orbital.
According to mot number of atomic orbitals combined is equal to total number of molecular orbitals formedelectronic configuration of h is 1s1. Here is the result obtained for egs r via eq. Discussed in this video are.
14 Linear Combination Of Atomic Orbitals Chemistry Libretexts
Sparknotes Molecular Orbitals Molecular Orbital Theory
Kinetic Energy And The Covalent Bond In H2
 Mo Theory H2 And H2 Solns Ppt Download
Mo Theory H2 And H2 Solns Ppt Download
Advanced Physical Chemistry Chem5350
 Solved Chapter 10 Problem 9fp Solution Principles Of Chemistry
Solved Chapter 10 Problem 9fp Solution Principles Of Chemistry
 Use The Molecular Orbital Diagram Shown To Clutch Prep
Use The Molecular Orbital Diagram Shown To Clutch Prep
Bohr S Hydrogen Molecule Ion H2
 Molecular Orbital Theory Build H2 Youtube
Molecular Orbital Theory Build H2 Youtube
 How Do You Write The Electron Configuration For H H 2 H 2
How Do You Write The Electron Configuration For H H 2 H 2
 Introduction To Molecular Orbital Theory
Introduction To Molecular Orbital Theory
 Determine The Structure Of Becl 2 Draw An Energy Level Diagram Of
Determine The Structure Of Becl 2 Draw An Energy Level Diagram Of
 Bonding In Homonuclear Diatomic Molecules Li2 Li2 Be2 B2 C2
Bonding In Homonuclear Diatomic Molecules Li2 Li2 Be2 B2 C2
 Dissociation And Ionization Of Quasi Periodically Vibrating H2 In
Dissociation And Ionization Of Quasi Periodically Vibrating H2 In
 Artificial Nodes In The H2 Wave Functions Expanded Using Gaussian
Artificial Nodes In The H2 Wave Functions Expanded Using Gaussian
Introduction To Molecular Orbital Theory
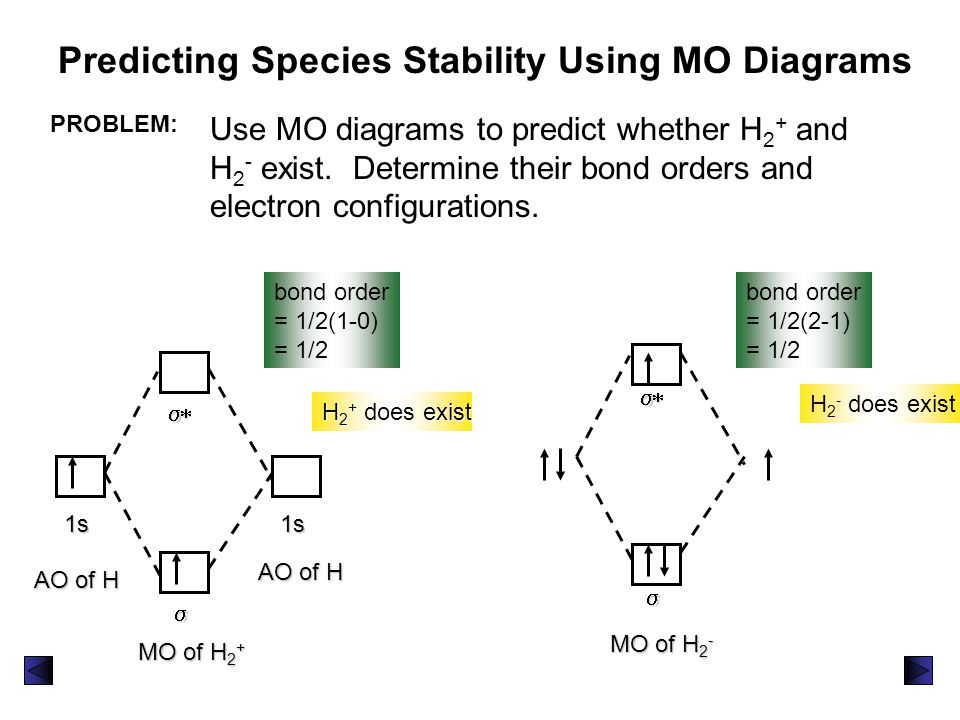 Molecular Orbital Theory Ppt Video Online Download
Molecular Orbital Theory Ppt Video Online Download
 Linear Combination Of Atomic Orbitals Wikipedia
Linear Combination Of Atomic Orbitals Wikipedia
 The Molecular Orbital Energy Level Diagrams For H2 H2 H2 And O2
The Molecular Orbital Energy Level Diagrams For H2 H2 H2 And O2

 Molecular Orbital Theory Ii Mo S Of The H2 Molecule Youtube
Molecular Orbital Theory Ii Mo S Of The H2 Molecule Youtube

0 Response to "H2 Molecular Orbital Diagram"
Post a Comment