Does This Diagram Represent An Increase Or Decrease In The Internal Energy Of The System
It represents a component of the gibbs free energy of that system. It may increase or decrease whether the process is reversible or irreversible is irrelevant because that may be determined by the other terms such as internal energy state u and pressure volume pv.
 Consider The Accompanying Energy Diagram Clutch Prep
Consider The Accompanying Energy Diagram Clutch Prep
Answer to visualizing conceptsconsider the accompanying energy diagram a does this diagram represent an increase or decrease in.
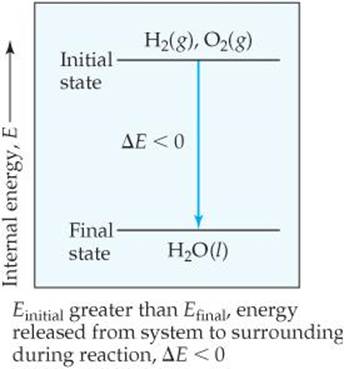
Does this diagram represent an increase or decrease in the internal energy of the system. Answer to does this diagram represent an increase or decrease in the internal energy of the system. C the system releases 575 kj of heat while doing 225 kj of work on the surroundings. A closed nonreactive system contains species 1 and 2 in vaporliquid equilibrium.
A does this diagram represent an increase or decrease in the internal energy of the system. Calculate delta e energy and determine whether the process is endothermic or exothermic for the follwing cases. Freezing water at below 0c being spontaneous change s universe 0 because the entropy increase in the surroundings is greater than the entropy decrease in the system.
A a system absorbs 105 kj of heat from its surroundings while doing 29 kj of work on the surroundings. As liquid water freezes heat is released which heats up the surroundings and increases the entropy of the surrounding change s surrounding 0. Species 2 is a very light gas essentially insoluble in the liquid phase.
A does this diagram represent an increase or decrease in the internal energy of the system. B q 150 kj and w 657 j. Does this diagram represent an increase or decrease in the internal energy of the system.
Increase decrease what sign i. Some additional moles of species 2 are added to the system which is then restored to its. Consider the accompanying energy diagram.
Use the order calculator below and get started. Heat or thermal energy symbolized by q is the energy transferred as a result of a difference in temperature between the system and the surroundings. For example energy in the form of heat is transferred from hot soup system to the bowl air and table surroundings because they are at a lower temperature.
The vapor phase contains both species 1 and 2. The area under a ts diagram has units of energy. Contact our live support team for any assistance or inquiry.

The First Law Of Thermodynamics
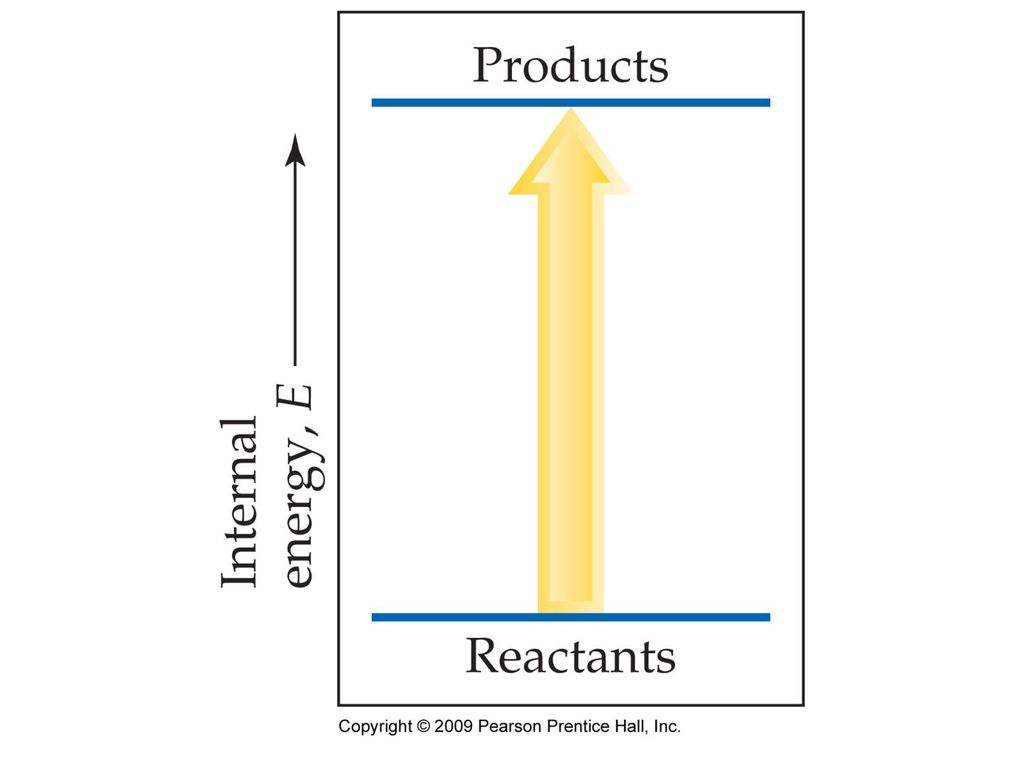 Figure Title Potential Energy And Kinetic Energy Caption Ppt
Figure Title Potential Energy And Kinetic Energy Caption Ppt
 The First Law Of Thermodynamics Thermochemistry Chemistry The
The First Law Of Thermodynamics Thermochemistry Chemistry The
The First Law Of Thermodynamics Closed Systems Heat Transfer
 For A Single Trajectory Internal Energy Does Not Increase
For A Single Trajectory Internal Energy Does Not Increase
 Why Do Gases Have Two Specific Heats Of Cp And Cv While Solids And
Why Do Gases Have Two Specific Heats Of Cp And Cv While Solids And
 What Is An Energy Flow Diagram Definition Applications
What Is An Energy Flow Diagram Definition Applications
Global Energy Transfer Atmosphere Climate
 The First Law Of Thermodynamics Physics
The First Law Of Thermodynamics Physics
 The Three Laws Of Thermodynamics Eagle Blog
The Three Laws Of Thermodynamics Eagle Blog
 Thermodynamics Part 1 Work Heat Internal Energy And Enthalpy
Thermodynamics Part 1 Work Heat Internal Energy And Enthalpy
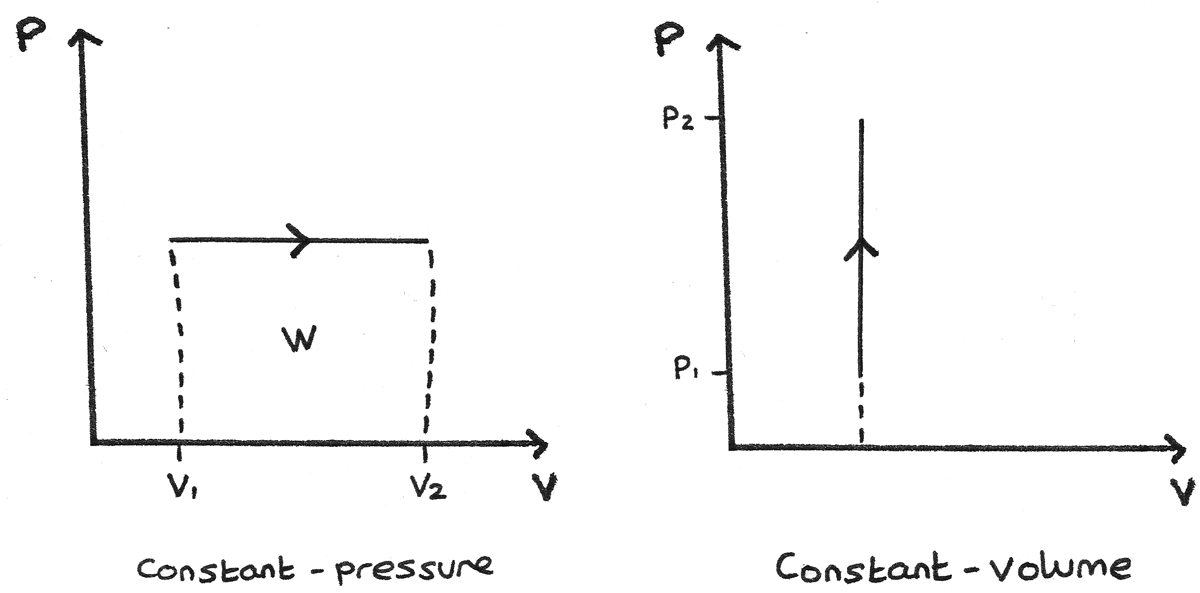 First Law And P V Diagrams Revise Im
First Law And P V Diagrams Revise Im
 Thermodynamics Internal Energy Of A System Can Be Increased Either
Thermodynamics Internal Energy Of A System Can Be Increased Either
Lecture Notes On Thermodynamics
 Energy Enthalpy And The First Law Of Thermodynamics
Energy Enthalpy And The First Law Of Thermodynamics
 A Level Physics Forces Fields And Energy Thermal Physics
A Level Physics Forces Fields And Energy Thermal Physics
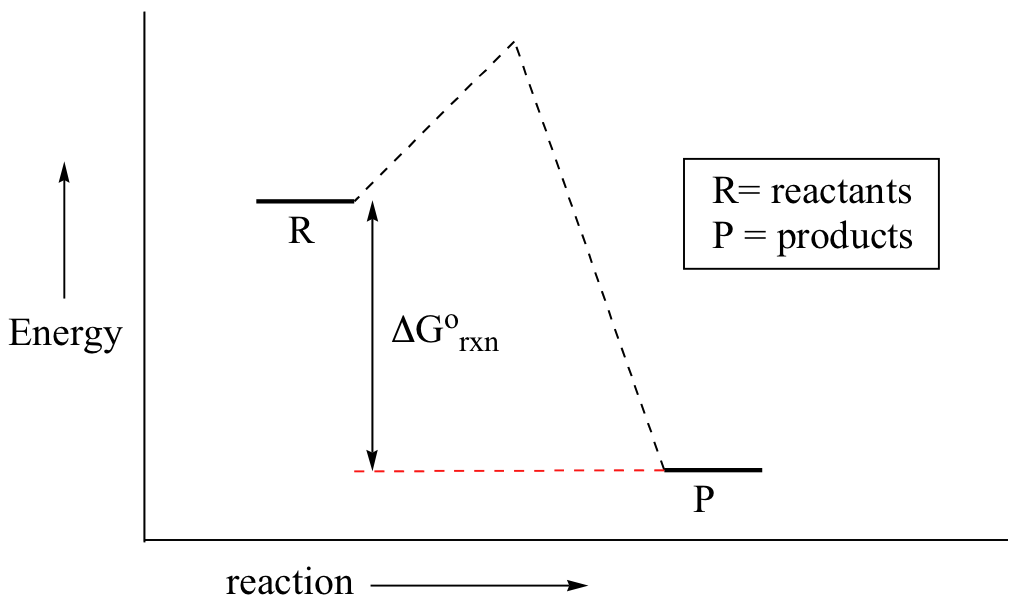 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
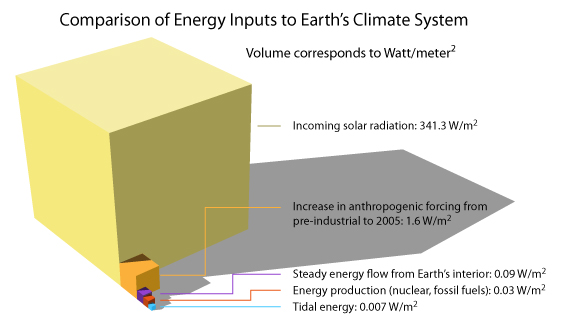 Heat From The Earth S Interior Does Not Control Climate
Heat From The Earth S Interior Does Not Control Climate
Refrigeration And Phase Changes
 How Does The Sun Produce Energy
How Does The Sun Produce Energy
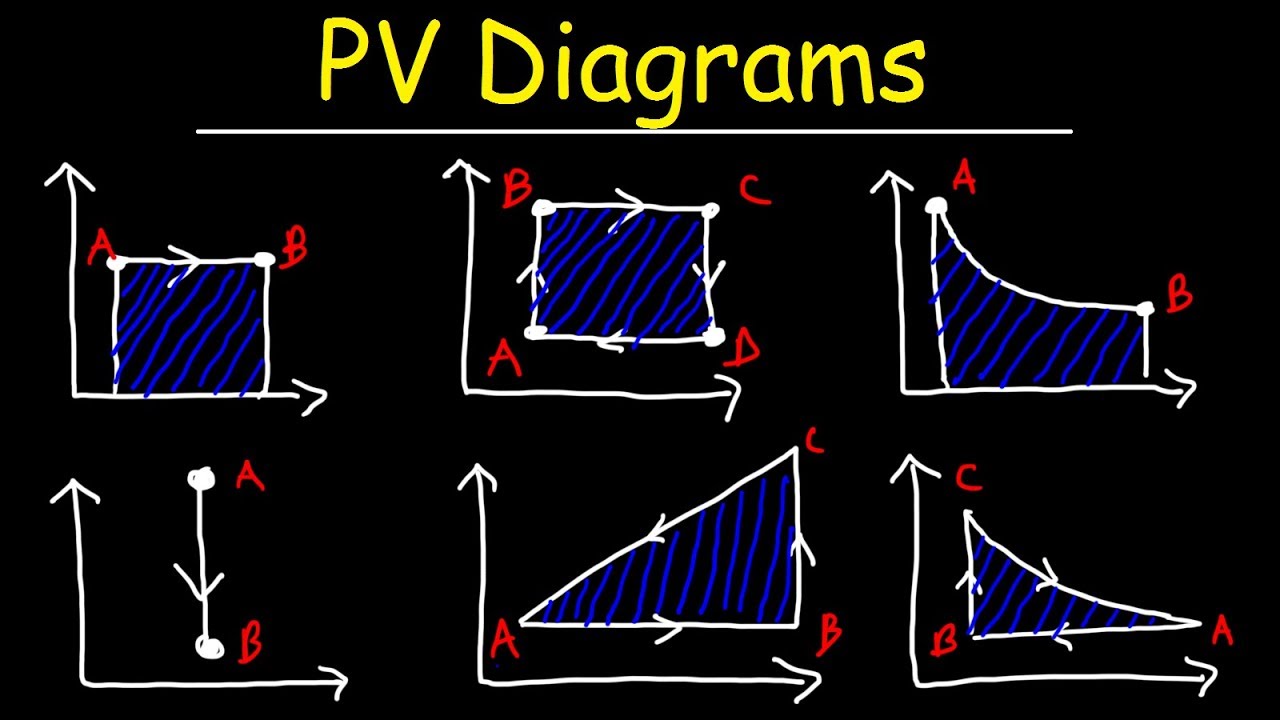 Pv Diagrams How To Calculate The Work Done By A Gas Thermodynamics
Pv Diagrams How To Calculate The Work Done By A Gas Thermodynamics
 Chapter 5 Thermochemistry Part 4 Of 11 Youtube
Chapter 5 Thermochemistry Part 4 Of 11 Youtube
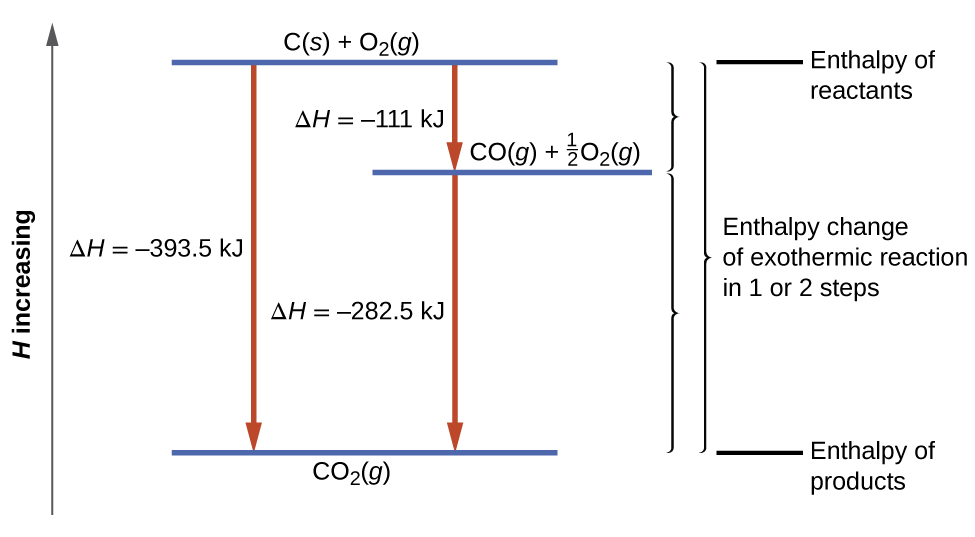
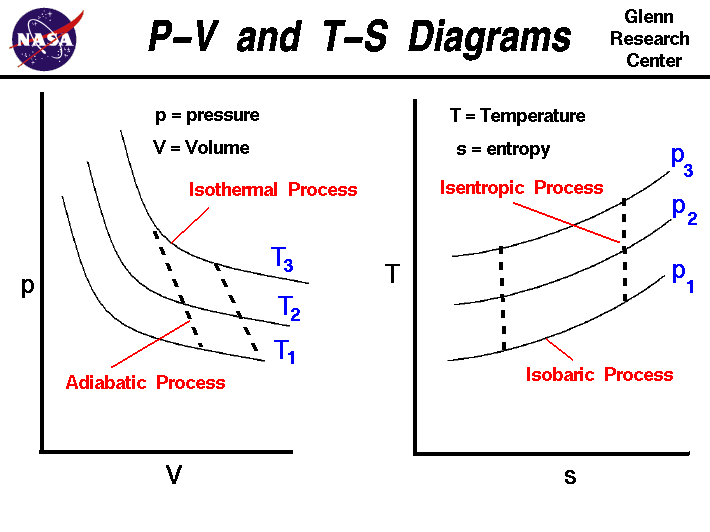

0 Response to "Does This Diagram Represent An Increase Or Decrease In The Internal Energy Of The System"
Post a Comment