What Is A Lone Pair In A Lewis Diagram
Lewis structures also known as lewis dot diagrams lewis dot formulas lewis dot structures electron dot structures or lewis electron dot structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. This video is my youtube premier.
They can be identified by using a lewis structure.

What is a lone pair in a lewis diagram. The lewis structure was named after gilbert n. On other hand the lone pair explains the basicity of the ammonia molecule. Lone pairs unpaired electrons and single double or triple bonds are used to indicate where the valence electrons are located around each atom in a lewis structure.
Ideally the three pairs of electrons should arrange themselves trigonally around the sn atom giving an angle of 120 between electron pairs and hence between the two cl atoms. Both the oxygen and the carbon now have an octet of electrons so this is an acceptable lewis electron structure. A double bond is represented by two solid lines drawn between a pair of atoms.
Lewis who introduced it in his 1916 article the atom and the molecule. Chemdraw 120 for the mac is used. The o has two bonding pairs and two lone pairs and c has four bonding pairs.
This is the structure of formaldehyde which is used in embalming fluid. If there are any bonds left over from step 3 create double bonds with lone pairs on outside atoms. Place remaining electrons around the central atom.
Most structuresespecially those containing second row elementsobey the octet rule in which every atom except h is surrounded by eight electrons. And they are normally represented by a double dotalternatively we could try to draw the sp3 hybrid orbital. Ammonium ion nh4 is a regular tetrahedron.
Complete the octet for the central atom with the remaining electrons. In chemistry a lone pair refers to a pair of valence electrons that are not shared with another atom and is sometimes called an unshared pair or non bonding pair. This chemistry video tutorial provides a basic introduction into drawing lewis dot structures but most importantly it provides an explanation on how to calculate the number of lone pairs using a.
A lewis structure can be drawn for any covalently bonded molecule as well as coordination compounds. If we draw the lewis diagram though we find a lone pair as well as two bonding pairs in the valence shell of the sn atom. Lone pairs are found in the outermost electron shell of atoms.
In it i will demonstrate how to add electron lone pairs as well as charges to atoms in lewis structures.
 Engr 1a Lecture Notes Fall 2018 Lecture 6 Linus Pauling Lone
Engr 1a Lecture Notes Fall 2018 Lecture 6 Linus Pauling Lone
 Draw The Lewis Structure For Hcn Include Lone Pairs
Draw The Lewis Structure For Hcn Include Lone Pairs
 Lewis Structure Molecular Geometry Atom Lone Pair Vsepr Theory Png
Lewis Structure Molecular Geometry Atom Lone Pair Vsepr Theory Png
 So32 Lewis Structure Wiring Diagram
So32 Lewis Structure Wiring Diagram
 Drawing Lewis Structures And Vsepr Draw Basic Lewis Dot Structures
Drawing Lewis Structures And Vsepr Draw Basic Lewis Dot Structures
 5 Which Of The Following Is The Correct Lewis Structure For Socl 2
5 Which Of The Following Is The Correct Lewis Structure For Socl 2
 13 Cpd Lewis Structure Atoms Lone Pairs Shape Sif 4 Consider The
13 Cpd Lewis Structure Atoms Lone Pairs Shape Sif 4 Consider The
 Draw The Lewis Structure Of Sf2 Showing All Lone Pairs Brainly Com
Draw The Lewis Structure Of Sf2 Showing All Lone Pairs Brainly Com
 5 Steps Lewis Dot Structure How To Do Draw Lewis Dot Structure
5 Steps Lewis Dot Structure How To Do Draw Lewis Dot Structure
 Solution Add Lone Pairs To These Lewis S Chemistry
Solution Add Lone Pairs To These Lewis S Chemistry
Illustrated Glossary Of Organic Chemistry Lewis Structure
65111 Chemistry 1 Lecture 8 Lewis Dots And Vsepr 065111
 Lewis Symbols And Structures Chemistry I
Lewis Symbols And Structures Chemistry I
 Nh3 Lewis Structure W Free Video Guide
Nh3 Lewis Structure W Free Video Guide
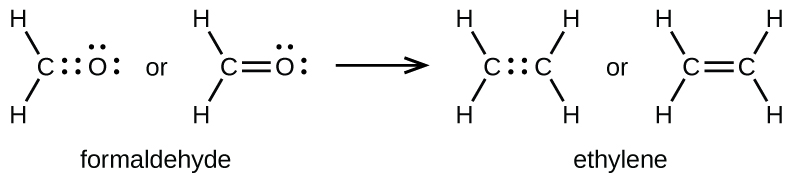 4 3 Lewis Structures Chemistry Libretexts
4 3 Lewis Structures Chemistry Libretexts
 In The Lewis Structure For So 2 How Many Lone Pairs Of Electrons Are
In The Lewis Structure For So 2 How Many Lone Pairs Of Electrons Are
 Lewis Dot Structures How To Calculate The Number Of Lone Pairs
Lewis Dot Structures How To Calculate The Number Of Lone Pairs
 In The Lewis Structure For Formic Acid Hcooh How Ma
In The Lewis Structure For Formic Acid Hcooh How Ma
 Lewis Structures In Organic Chemistry Chemistry Steps
Lewis Structures In Organic Chemistry Chemistry Steps
Chem 2303 Supplementary Problems
 Solutions To Chem 1 Exams 1996 97 2nd Midterm
Solutions To Chem 1 Exams 1996 97 2nd Midterm
How To Recognizing Implicit Lone Pairs In Abbreviated Lewis Structures
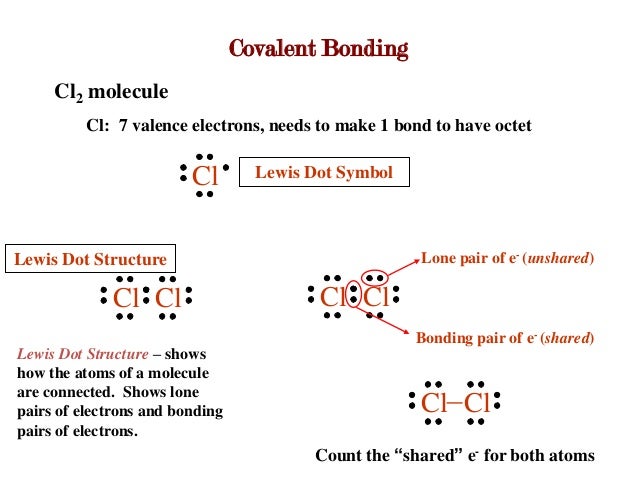
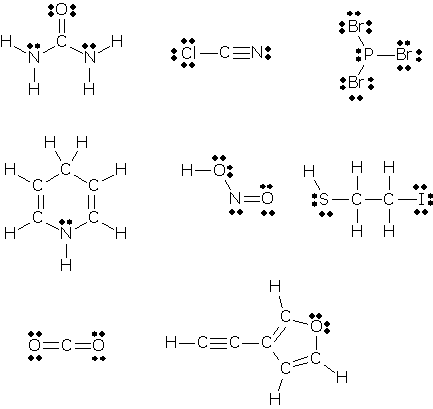
0 Response to "What Is A Lone Pair In A Lewis Diagram"
Post a Comment