Molecular Orbital Diagram Of Benzene
The calculated energy of the molecular orbital is shown in the left corner. Lets look at an energy diagram of the pi molecular orbitals in benzene.
 15 3 Pi Molecular Orbitals Of Benzene Chemistry Libretexts
15 3 Pi Molecular Orbitals Of Benzene Chemistry Libretexts
Quantum mechanical calculations tell us that the six pi molecular orbitals in benzene formed from six atomic p orbitals occupy four separate energy levels.
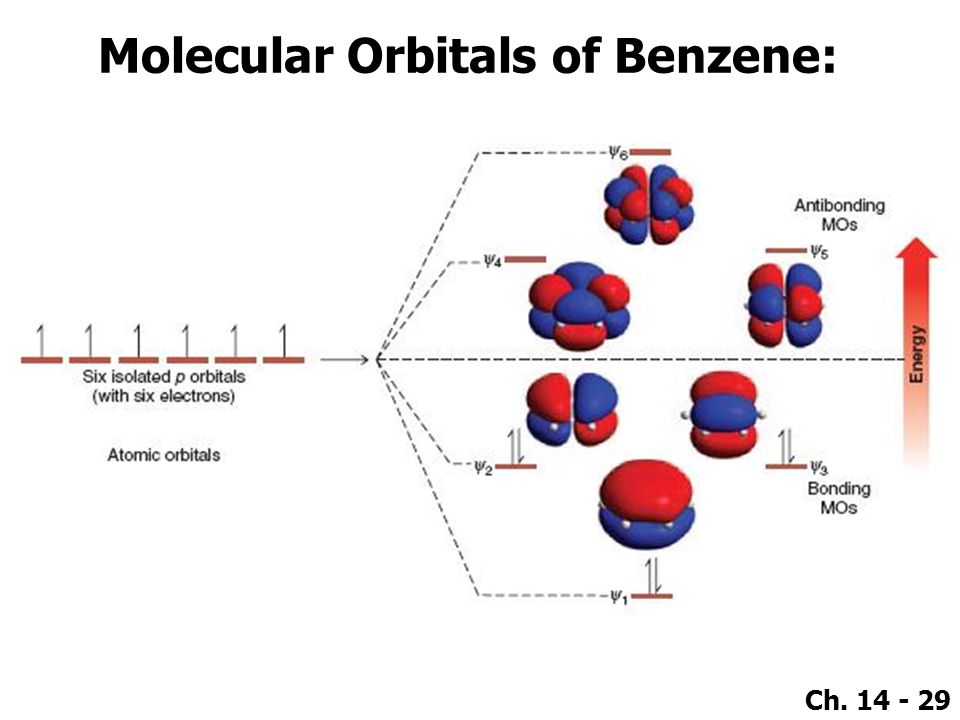
Molecular orbital diagram of benzene. It reiterates many of the concepts we have discussed already in terms of the mos of conjugated systems including. Following the pattern described above for butadiene. Two sp 2 hybrid orbitals of each c atom overlap with two sp 2 hybrid orbital of two other c atoms to form sigma bonds.
Hexatriene and benzene each have six pi molecular orbitals. The valence orbitals are the 6 carbon 2p z orbitals. Use the buttons to display the sp 2 orbitals that make up the sigma framework and the remaining p orbitals which form the pi bonding orbital.
Two electrons occupy the 1s orbital. Three electrons participate in sp² bonds with the neighboring carbon atoms or with a hydrogen atom. However all of the carbon carbon bonds in the benzene molecule are of the same length and it is known that a single bond is longer than a double bond.
This video illustrates deep molecular orbital analyses of benzene and hexatriene. Carbon has 6 electrons. Bonding orbitals in benzene sp 2.
Molecular orbitals of benzene. In addition the bond length the distance between the two bonded atoms in benzene is greater than a double bond but shorter than a single bond. The sixth electron occupies the 2p z orbital which is half filled.
Pi 1 and pi 6 have unique energy levels while the pi 2 pi 3 and pi 4 pi 5 pairs are degenerate. Explore bonding orbitals in other small molecules hydrogen fluorine nitrogen hydrogen fluoride carbon monoxide methane ammonia ethylene. π molecular orbitals of benzene.
Note that the figure showing the molecular orbitals of benzene has two bonding π 2 and π 3 and two anti bonding π and π 5 orbital pairs at the same energy levels. The pi molecular orbitals of benzene 1. Orbitals with the same energy are described as degenerate orbitals.
Drawing the ground floor. The structure of benzene molecule is best described in terms of molecular orbital treatment theory. The highest energy molecular orbitals have p orbitals.
According to this theory all the c atoms in benzene are sp 2 hybridized.
 Introduction To Molecular Orbital Theory
Introduction To Molecular Orbital Theory
 Sparknotes Organic Chemistry Orbitals Molecular Orbital Theory
Sparknotes Organic Chemistry Orbitals Molecular Orbital Theory
 The Many Guises Of Aromaticity American Scientist
The Many Guises Of Aromaticity American Scientist
 Ppt 15 Benzene And Aromaticity Powerpoint Presentation Id 6585746
Ppt 15 Benzene And Aromaticity Powerpoint Presentation Id 6585746
 In Chemistry What Are Resonance Structures Quora
In Chemistry What Are Resonance Structures Quora
 Frost Circles And How To Use Them Master Organic Chemistry
Frost Circles And How To Use Them Master Organic Chemistry
 Frost Circles And How To Use Them Master Organic Chemistry
Frost Circles And How To Use Them Master Organic Chemistry
 2 2 Molecular Orbital Theory Conjugation And Aromaticity
2 2 Molecular Orbital Theory Conjugation And Aromaticity
 Molecular Orbital Theory What Is Responsible For Making The Bonds
Molecular Orbital Theory What Is Responsible For Making The Bonds
 How Many Sigma Bonds Are In Benzene Socratic
How Many Sigma Bonds Are In Benzene Socratic
 Huckel S Molecular Orbital Theory By Sean Hanley Ppt Video Online
Huckel S Molecular Orbital Theory By Sean Hanley Ppt Video Online
 Stability Of Benzene Molecular Orbital Theory Mot In Hindi
Stability Of Benzene Molecular Orbital Theory Mot In Hindi
 Chapter 14 Aromatic Compounds Ppt Video Online Download
Chapter 14 Aromatic Compounds Ppt Video Online Download
 Discuss The Molecular Orbital Structure Of Benzene Delocalisation
Discuss The Molecular Orbital Structure Of Benzene Delocalisation
 Chemistry Molecular Structure 41 Of 45 Delocalized Molecular
Chemistry Molecular Structure 41 Of 45 Delocalized Molecular
 Molecular Orbital Treatment Of Benzene Bond Lingth Analysis In
Molecular Orbital Treatment Of Benzene Bond Lingth Analysis In
 Aromatic Compounds Overview Chemgapedia
Aromatic Compounds Overview Chemgapedia
 Chem 282 Textbook Notes Fall 2015 Chapter 14 6 Unpaired
Chem 282 Textbook Notes Fall 2015 Chapter 14 6 Unpaired
 Benzene Structure Chemistry Tutorvista Com
Benzene Structure Chemistry Tutorvista Com
 The Pi Molecular Orbitals Of Benzene Master Organic Chemistry
The Pi Molecular Orbitals Of Benzene Master Organic Chemistry
 Solved According To Molecular Orbital Theory How Many
Solved According To Molecular Orbital Theory How Many
 Introduction To Molecular Orbital Theory
Introduction To Molecular Orbital Theory
 Energy Level Diagram And Schematic Representation Of The P Molecular
Energy Level Diagram And Schematic Representation Of The P Molecular
 P Molecular Orbitals Of Conjugated Butadiene
P Molecular Orbitals Of Conjugated Butadiene
 Part 2 7 Orbital Diagrams Ppt Download
Part 2 7 Orbital Diagrams Ppt Download
 Mo16 Aromatics Chemistry Libretexts
Mo16 Aromatics Chemistry Libretexts
 Benzene Introduction To Aromatic Compounds Chemgapedia
Benzene Introduction To Aromatic Compounds Chemgapedia
 11 6 Delocalized Electrons Bonding In The Benzene Molecule
11 6 Delocalized Electrons Bonding In The Benzene Molecule
 The Most Stable Mo Of 1 3 5 Hexatriene And The Most Stable Studysoup
The Most Stable Mo Of 1 3 5 Hexatriene And The Most Stable Studysoup


0 Response to "Molecular Orbital Diagram Of Benzene"
Post a Comment