How Is Activation Energy Represented On An Energy Diagram
In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. 1 activation energy is greater for endothermic reactions.
Activation energy is the same for both endothermic and exothermic reactions.
How is activation energy represented on an energy diagram. The change of enthalpy of the reaction equals the difference of the activation energy of forward and reverse reaction. It is associated with the formation of the activated complex from the reactants. δhr e e because the is no change in the number of moles volume stays constant throughout reaction and change of enthalpy equals the change of energy.
At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products. In the energy diagram of an endothermic reaction the products side is lower than the reactants side. However it does not change the potential energy of the reactants or products.
Since the activation energy is the highest point of an energy diagram it would take a longer time for. The activation energy diagram is drawn as a hill because there is a large amount of energy needed to form the unstable transition state viewed as the upward slope on the diagram. Activation energy for the forward reaction.
The minimum energy required to convert reactants into products. Activation energy is greater for endothermic reactions. The difference between the energies of the activated complex and the reactants heat of reaction.
It also does not affect the heat. Activation energy is greater for exothermic reactions. Activation energy is represented by a negative slope.
The difference of potential energy between the reactants and products. On a potential energy diagram what is true about activation energy. A catalyst lowers the activation energy for both the forward and reverse reaction.
This state is also known as an activated complex. Activation energy is the energy that molecules have to possess for a reaction to occur.
 A Look At Energy Profiles For Reactions Chemistry Libretexts
A Look At Energy Profiles For Reactions Chemistry Libretexts
 E Kissinger Plots Representing The Activation Energy E A Of Mgh 2
E Kissinger Plots Representing The Activation Energy E A Of Mgh 2
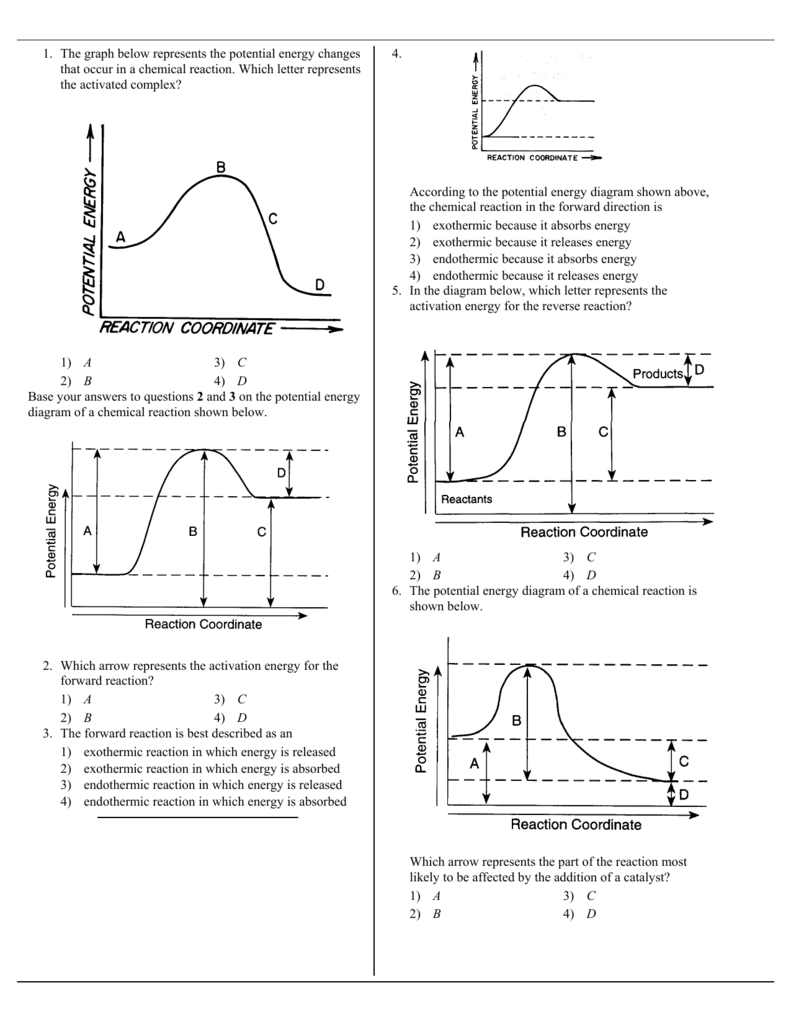
 1 The Graph Below Represents The Potential Energy Changes That
1 The Graph Below Represents The Potential Energy Changes That
 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
 How To Read Potential Energy Diagrams
How To Read Potential Energy Diagrams
 Solved The Reaction Energy Diagrams For An Endothermic An
Solved The Reaction Energy Diagrams For An Endothermic An
 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
 1 The Graph Below Represents The Potential Energy
1 The Graph Below Represents The Potential Energy
 Which Reaction Coordinate Diagram Represen Clutch Prep
Which Reaction Coordinate Diagram Represen Clutch Prep
 Activation Energies And Uncertainty Lesswrong 2 0
Activation Energies And Uncertainty Lesswrong 2 0
 Activation Energy Article Enzymes Khan Academy
Activation Energy Article Enzymes Khan Academy
 Oranbind Enzymes And Activation Energy Graph Questions
Oranbind Enzymes And Activation Energy Graph Questions
 Activation Energy Read Chemistry Ck 12 Foundation
Activation Energy Read Chemistry Ck 12 Foundation
 Activation Energy Brilliant Math Science Wiki
Activation Energy Brilliant Math Science Wiki
 How Can I Represent The Activation Energy In A Potential Energy
How Can I Represent The Activation Energy In A Potential Energy
 Solved Which Of The Following Energy Diagrams 1 2 3 Or
Solved Which Of The Following Energy Diagrams 1 2 3 Or
 How Can I Represent An Exothermic Reaction In A Potential Energy
How Can I Represent An Exothermic Reaction In A Potential Energy
 Potential Energy Diagrams For A Given Reaction The Activation
Potential Energy Diagrams For A Given Reaction The Activation
 12 2 Factors Affecting Reaction Rates Chemistry
12 2 Factors Affecting Reaction Rates Chemistry
 1 The Graph Below Represents The Potential Energy Changes That
1 The Graph Below Represents The Potential Energy Changes That
 What Is The Activation Energy For A Reverse Reaction Quora
What Is The Activation Energy For A Reverse Reaction Quora
 Aim What Factors Affect Rate Of Reactions Ppt Download
Aim What Factors Affect Rate Of Reactions Ppt Download
 Solved H3 17 The Potential Energy Diagram Below Represent
Solved H3 17 The Potential Energy Diagram Below Represent
 1 The Graph Below Represents The Potential Energy Changes That
1 The Graph Below Represents The Potential Energy Changes That
 A Look At Energy Profiles For Reactions Chemistry Libretexts
A Look At Energy Profiles For Reactions Chemistry Libretexts
 Chapter 8 Quiz Questions At University Of Massachusetts Boston
Chapter 8 Quiz Questions At University Of Massachusetts Boston
 Potential Energy Diagram Worksheet Part I
Potential Energy Diagram Worksheet Part I
 Igcse Chemistry 4 14 Represent Exothermic And Endothermic Reactions
Igcse Chemistry 4 14 Represent Exothermic And Endothermic Reactions
 Vi Kinetics Equilibrium Ppt Download
Vi Kinetics Equilibrium Ppt Download



0 Response to "How Is Activation Energy Represented On An Energy Diagram"
Post a Comment