Sketch The Phase Diagram To Answer Whether Solid Argon Or Liquid Argon Has The Greater Density
B describe any chances that can be observed in a sample of solid argon when. Its critical temperature is 1508 k and critical pressure is 483 atm.
 Phase Equilibrium And Solutions
Phase Equilibrium And Solutions
Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density.
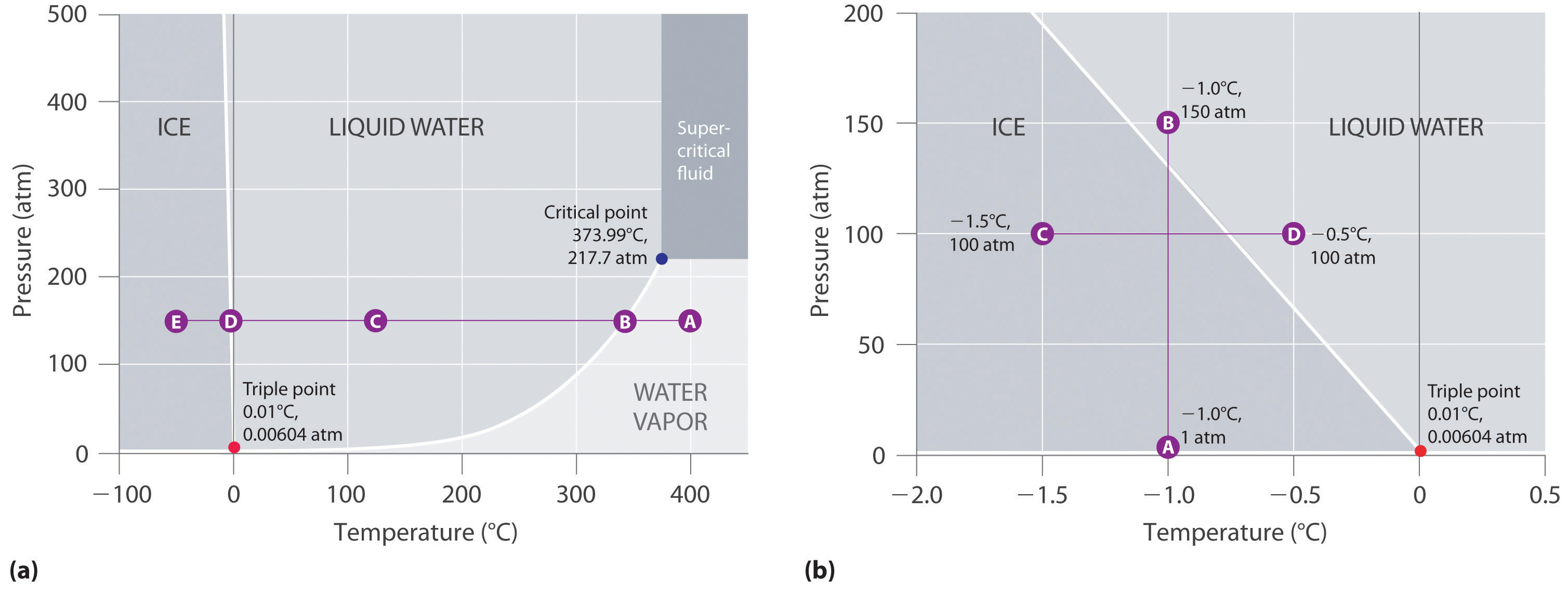
Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density. Part a sketch the phase diagram to answer whether solid argon or liquid argon has the greater density. Its critical temperature is 1508k and critical pressure is 483 atm. Calculate the vapor pressure of a solution containing 245 g of glycerin c3h8o3 in 135 ml of water at 300c.
You have to go to about 200 degrees centigrate to make oxygen and nitrogen turn to. It has a triple point at 837 k and 068 atm. Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density.
It has a triple point at 837 and 068. A use the data above to draw a phase diagram for argon. On the phase diagram show the position of the normal boiling point.
Argon has a normal boiling point of 872 k and a melting point at 1 atm of 841 k. Label the axes and label the regions in which the solid liquid and gas phases are stable. Its critical temperature is 1262k and critical pressure is 255 x 104 torr.
Correct exercise 1190 the high pressure phase diagram of ice is shown at the top of the next column. A solid object in contact with a liquid in which the solid does not dissolve will sink if the density of the solid is greater than the density of the liquid and will float if the density of the. Mostly solid nitrogen quite a lot of solid oxgen a bit of solid argon and some solid carbon dioxide and ice.
Argon has a normal boiling point of 872k and a melting point at 1 atm of 841k. The vapor pressure of pure water at this temperature is 318 torr. Argon has a normal boiling point of 872 k and a melting point at 1 atm of 841 k.
A mixture containing 224 g of ice at exactly 000 c and 742 g of water at 588 c is placed in an insulated container. It has a triple point at 837 k and 068 atmsketch the phase diagram to answer whether solid argon or liquid argon has the greater densit. Notice that under high pressure ice can exist in several different solid forms.
It has a triple point at 837k and 068 atm. Its critical temperature is 1508 k and critical pressure is 483 atm. Which has a higher density liquid or solid argon.
100 11 ratings or. See the answer nitrogen has a normal boiling point of 773 k and a melting point at 1atm of 631 k. Assume that glycerin is not volatile and dissolves molecularly not ionically and use a density of 100 gml for the water.
 5 E Exercises Chemistry Libretexts
5 E Exercises Chemistry Libretexts
 Solution Based On The Phase Diagram Show Chemistry
Solution Based On The Phase Diagram Show Chemistry
 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry
 Nitrogen Has A Normal Boiling Point Of 77 Clutch Prep
Nitrogen Has A Normal Boiling Point Of 77 Clutch Prep
 Solved Argon Has A Normal Boiling Point Of 87 2 K And A Meltin
Solved Argon Has A Normal Boiling Point Of 87 2 K And A Meltin
 Liquid Water Denser Than Solid Water Ice Video Khan Academy
Liquid Water Denser Than Solid Water Ice Video Khan Academy
 5 E Exercises Chemistry Libretexts
5 E Exercises Chemistry Libretexts
 Solved Argon Gas Has Its Triple Point At 189 3 C And 516 Mm H
Solved Argon Gas Has Its Triple Point At 189 3 C And 516 Mm H
 Ap Chemistry Problem Set Chapter 10 Name Multiple
Ap Chemistry Problem Set Chapter 10 Name Multiple
 Intermolecular Forces Liquids And Solids
Intermolecular Forces Liquids And Solids
 Phase Diagram Chemistry Video Clutch Prep
Phase Diagram Chemistry Video Clutch Prep
 Intermolecular Forces Liquids And Solids
Intermolecular Forces Liquids And Solids
 5 E Exercises Chemistry Libretexts
5 E Exercises Chemistry Libretexts
 Phase Equilibrium And Solutions
Phase Equilibrium And Solutions
 Gases And Imfs Unit Exam Review Guide
Gases And Imfs Unit Exam Review Guide
 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Ap Chemistry Problem Set Chapter 10 Name Multiple
Ap Chemistry Problem Set Chapter 10 Name Multiple
 Heat Energy And The States Of Matter
Heat Energy And The States Of Matter
 Phase Equilibrium And Solutions
Phase Equilibrium And Solutions
 Ap Questions Imf S And Phase Diagrams B For Any Pairs Of
Ap Questions Imf S And Phase Diagrams B For Any Pairs Of
 Molecular Interactions Noncovalent Interactions
Molecular Interactions Noncovalent Interactions
 Phase Diagram Chemistry Video Clutch Prep
Phase Diagram Chemistry Video Clutch Prep
 Chemistry 1412 2 Chapter 11 Flashcards Quizlet
Chemistry 1412 2 Chapter 11 Flashcards Quizlet
 Solved Indicate Whether The Average Kinetic Energy Of The
Solved Indicate Whether The Average Kinetic Energy Of The
 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry



0 Response to "Sketch The Phase Diagram To Answer Whether Solid Argon Or Liquid Argon Has The Greater Density"
Post a Comment