The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Atom
N infinity match each arrow with the correct response below. This chemistry video tutorial focuses on the bohr model of the hydrogen atom.
 The Following Is A Diagram Of Energy States And Tr Chegg Com
The Following Is A Diagram Of Energy States And Tr Chegg Com
The 2s and 2p states are found to differ a small amount in what is called the lamb shift.
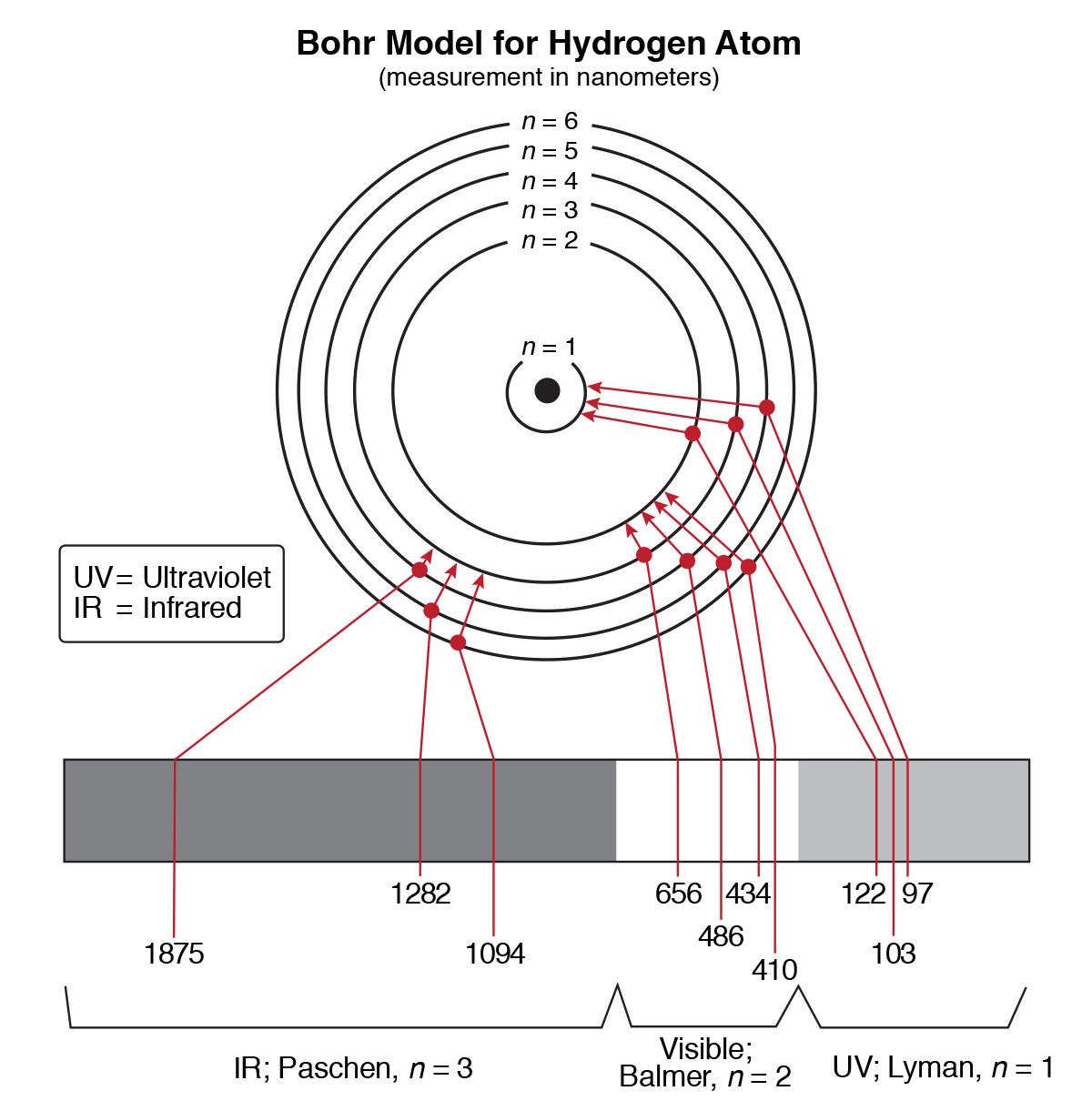
The following is a diagram of energy states and transitions in the hydrogen atom. Hydrogen energy level plot. In other words the wavelength λ can only take on specific values since n1 and n2 are integers. The labeled transitions a through e represent an electron moving between energy levels.
Because an electron bound to an atom can only have certain energies the electron can only absorb photons of certain energies exactly matched to the energy difference or quantum leap between two energy states. The following is a diagram of energy states and transitions in the hydrogen atom. For hydrogen the ionization energy 136ev when an excited electron returns to a lower level it loses an exact amount of energy by emitting a photon.
The following is a diagram of energy states and transitions in the hydrogen atom. The emission line with the longest wavelength. And even the 1s ground state is split by the interaction of electron spin and nuclear spin in what is called hyperfine structure.
The 2p level is split into a pair of lines by the spin orbit effect. The following is a diagram of energy states and transitions in the hydrogen atom. The emission line with the highest energy.
The following diagram represents energy levels in a hydrogen atom. The following is a diagram of energy states and transitions in the hydrogen atom. The absorption line with the longest wavelength.
The absorption line with the highest energy. Figure 1 if an electron at level 1 in a hydrogen atom absorbs 102 ev of energy it moves to level 2. Match each arrow with the correct response below.
Match each arrow with the correct response below. It is common convention to say an unbound electron has zero binding energy. 1 the emission line with the longest wavelength 2 the absorption line with the longest wavelength 3.
Since the energy level of the electron of a hydrogen atom is quantized instead of continuous the spectrum of the lights emitted by the electron via transition is also quantized. Match each of the responses below with the correct arrow from the figure. Since the final energy level is higher than the.
Transition of an electron and spectral lines. The ionization energy of an atom is the energy required to remove the electron completely from the atomtransition from ground state n 0 to infinity n. B the absorption line with the shortest wavelength.
4 a the emission line with the shortest wavelength. Since the final energy level is higher than the initial level the electron is further from the nucleus. It explains how to calculate the amount of electron transition energy that is released or absorbed whenever an.
Energy level diagram for hydrogen. A the emission line with the shortest wavelength.
 Draw A Neat And Labelled Energy Level Diagram And Explain Balmer
Draw A Neat And Labelled Energy Level Diagram And Explain Balmer
 5 7 Spectral Lines Of Atomic Hydrogen Chemistry Libretexts
5 7 Spectral Lines Of Atomic Hydrogen Chemistry Libretexts
 Introduction To Molecular Orbital Theory
Introduction To Molecular Orbital Theory
 Chapter 6 Lecture Electrons In Atoms
Chapter 6 Lecture Electrons In Atoms
 Electron Transitions And Formation Of The Spectra Chapter 2
Electron Transitions And Formation Of The Spectra Chapter 2
 Atomic Theory 1 33 The Hydrogen Spectrum
Atomic Theory 1 33 The Hydrogen Spectrum
 Solved The Following Is A Diagram Of Energy States And Tr
Solved The Following Is A Diagram Of Energy States And Tr
 Draw A Neat Labelled Energy Level Diagram For H Atom Showing The
Draw A Neat Labelled Energy Level Diagram For H Atom Showing The
 Hydrogen Spectrum Activity Carolina Com
Hydrogen Spectrum Activity Carolina Com
 Observation Of The 1s 2p Lyman A Transition In Antihydrogen Nature
Observation Of The 1s 2p Lyman A Transition In Antihydrogen Nature
 Nonradiative Transition An Overview Sciencedirect Topics
Nonradiative Transition An Overview Sciencedirect Topics
 Atomic Hydrogen Emission Spectrum
Atomic Hydrogen Emission Spectrum
 Page 1 984 Chapter 29 Atoms And Molecules Summary The Goal
Page 1 984 Chapter 29 Atoms And Molecules Summary The Goal
 Dft Calculated Structures Of The Energy Minimum And The Transition
Dft Calculated Structures Of The Energy Minimum And The Transition
 Hydrogen Energies And Spectrum
Hydrogen Energies And Spectrum
 Scheme Of Levels Of Energy Of Hydrogen Atom The Vertical Arrows
Scheme Of Levels Of Energy Of Hydrogen Atom The Vertical Arrows
 Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy
Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy
 8 3 Electron Configurations How Electrons Occupy Orbitals
8 3 Electron Configurations How Electrons Occupy Orbitals
 Spectroscopy Chapter 3 Spectroscopy And Photochemistry Of
Spectroscopy Chapter 3 Spectroscopy And Photochemistry Of
 Emission And Absorption Spectra Optical Phenomena And Properties
Emission And Absorption Spectra Optical Phenomena And Properties
 The Rydberg Constant And Proton Size From Atomic Hydrogen Science
The Rydberg Constant And Proton Size From Atomic Hydrogen Science
 Solved The Following Is A Diagram Of Energy States And Tr
Solved The Following Is A Diagram Of Energy States And Tr



0 Response to "The Following Is A Diagram Of Energy States And Transitions In The Hydrogen Atom"
Post a Comment