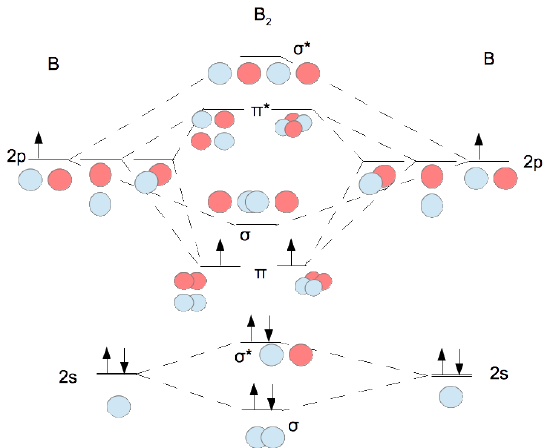
B2 2 Molecular Orbital Diagram
A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. A number of.
Molecular orbital diagrams of diatomic molecules introduction.
.png)
B2 2 molecular orbital diagram. Molecular orbital diagram for beryllium dimer be2 fill from the bottom up with 4 electrons total. The electronic configuration of b atom z 5 is. View a full sample.
Since bond order is zero be 2 molecule does not exist. This video shows the end of the be2 molecule mo diagram and explains pi orbitals paramagnetism and the mo diagrams for b2. Why is this not the case in the ceb2 mo.
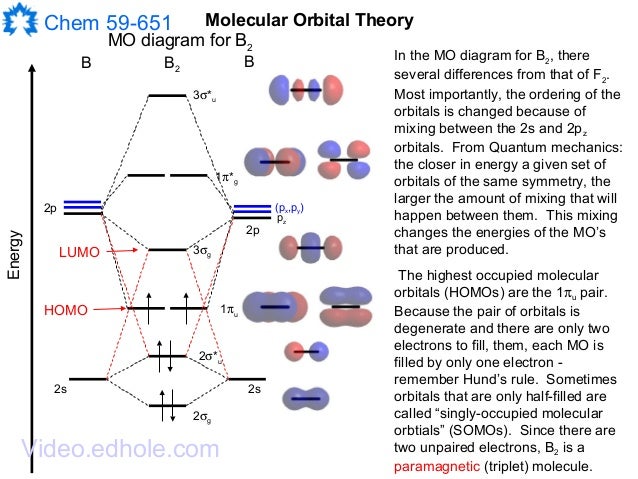
The molecular orbital electronic configuration magnetic property. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. In chemistry molecular orbital mo theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms but are treated as moving under the influence of the nuclei in the whole molecule.
It is diamagnetic due to the absence of any unpaired electron. Additionally i labelled the humo as pi2py and the lumo as sigma2px. I drew a diagram of b2 in which i filled both bonding and anti bonding orbitals of 2s sigma and 2s sigma.
As well i filled in the sigma 2px and pi 2py orbits. Atomic orbitals and molecular orbitals of a molecule can be shown in a molecular orbital diagram. Comment0 chapter problem is solved.
This was on a quiz and i somehow got the bond order and the lumo indicated wrong. The molecular orbital diagram for ceo2 says that the sigma 2p bonding molecular orbital is lower in energy than the pi 2p bonding molecular orbital. Why is there a difference between o2 and b2 sigma 2p molecular orbitals in diagrams.
I also calculated the bond order of this molecule to be 32. View a sample solution. Use mo diagrams to place b2b2 and b2 in order ofa decr.
A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. Bonding order is 0 meaning it does not bond and it is diamagnetic.
.png) In B2 Is Highest Occupied Molecular Orbital Is Of Type
In B2 Is Highest Occupied Molecular Orbital Is Of Type
 Please Tell Me Why In B2 C2 And N2 In P Orbital Order Is Taken As
Please Tell Me Why In B2 C2 And N2 In P Orbital Order Is Taken As
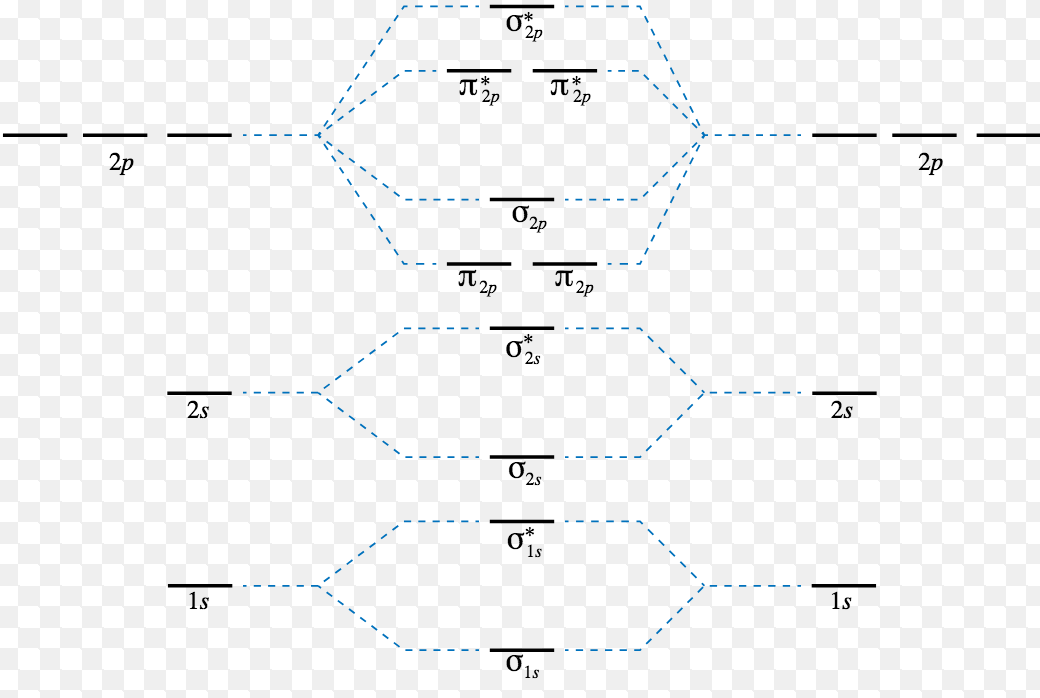 Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Energy Level Diagram For Molecular Orbitals Chemical Bonding And
 Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Energy Level Diagram For Molecular Orbitals Chemical Bonding And
What Is The Molecular Orbital Diagram For B 2 Socratic
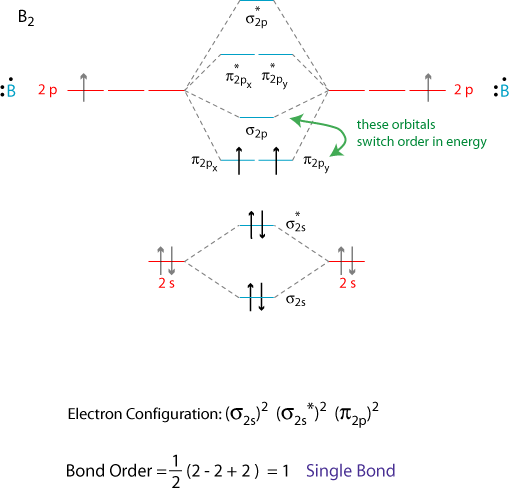 Molecular Orbital Theory Grandinetti Group
Molecular Orbital Theory Grandinetti Group
Mo Diagram For Boron Wiring Diagram Mega
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
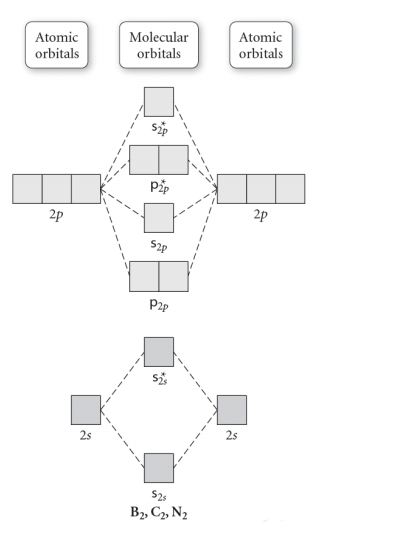 Use The Molecular Orbital Diagram Shown To Determine
Use The Molecular Orbital Diagram Shown To Determine
 Part 2 7 Orbital Diagrams Ppt Download
Part 2 7 Orbital Diagrams Ppt Download
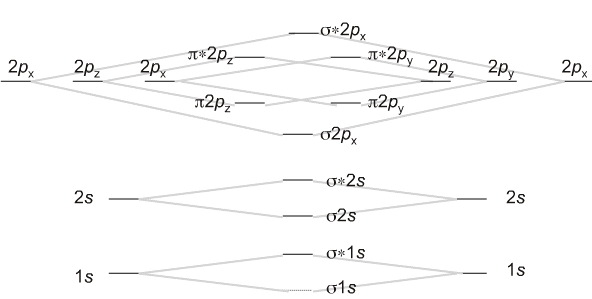 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 Bonding In Homonuclear Diatomic Molecules Li2 Li2 Be2 B2 C2
Bonding In Homonuclear Diatomic Molecules Li2 Li2 Be2 B2 C2
Mo Diagrams For Diatomic Molecules
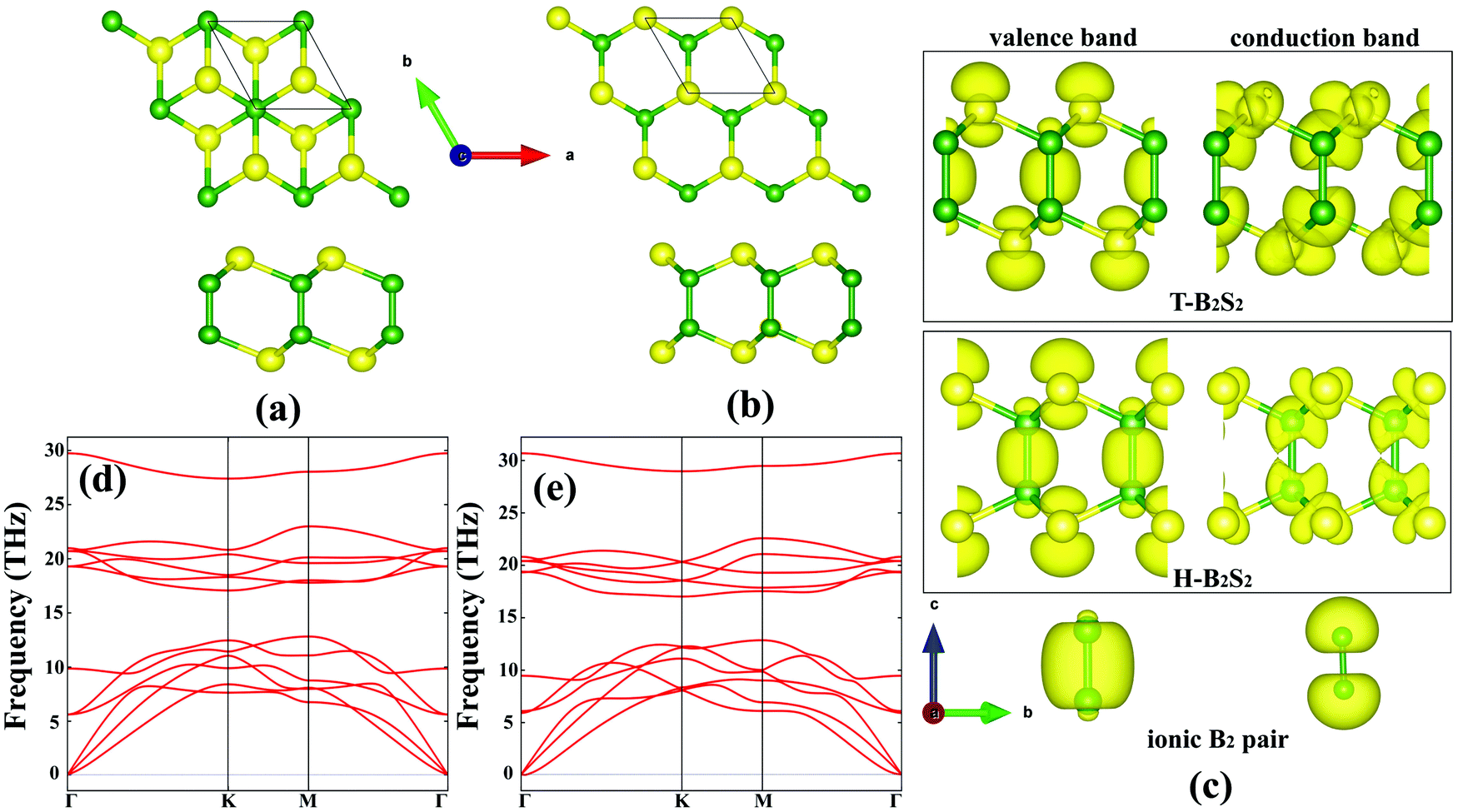 2d Boron Dichalcogenides From The Substitution Of Mo With Ionic B2
2d Boron Dichalcogenides From The Substitution Of Mo With Ionic B2
Chem 2303 Supplementary Problems
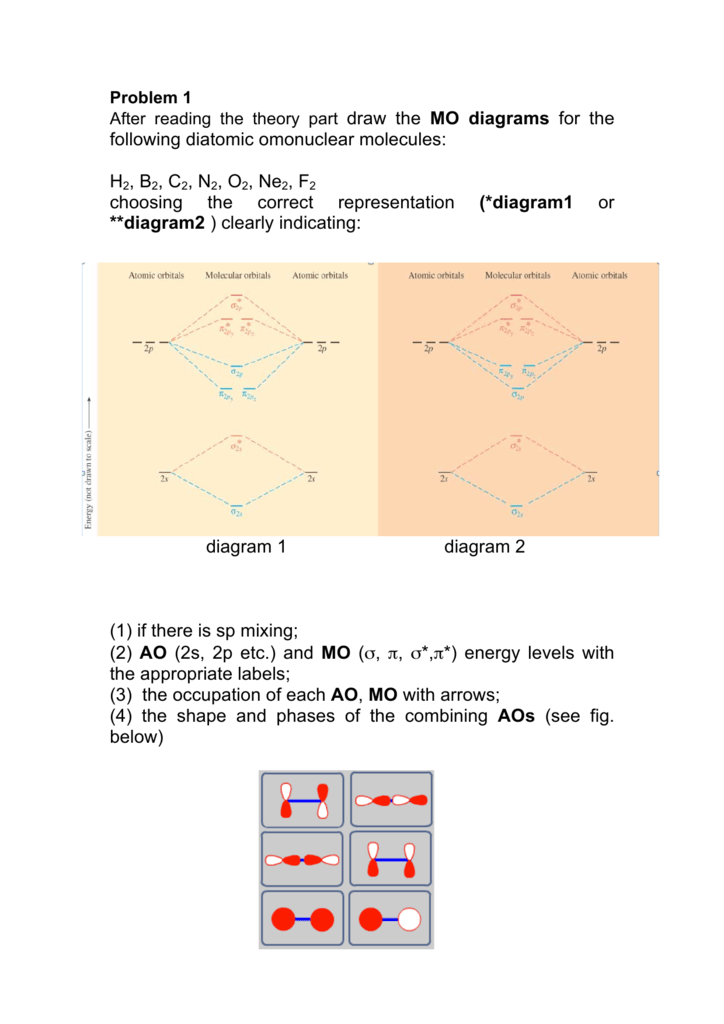 Following Diatomic Omonuclear Molecules H2 B2 C2 N2 O2 Ne2
Following Diatomic Omonuclear Molecules H2 B2 C2 N2 O2 Ne2
 Multiple Bonds Bond Energies Lengths Molecular Orbital Theory
Multiple Bonds Bond Energies Lengths Molecular Orbital Theory

Principles Of Chemical Science Solutions For Lecture 13 Molecular
Orbital Interaction Diagram 1 Plot Atomic Valence Orbital Energies
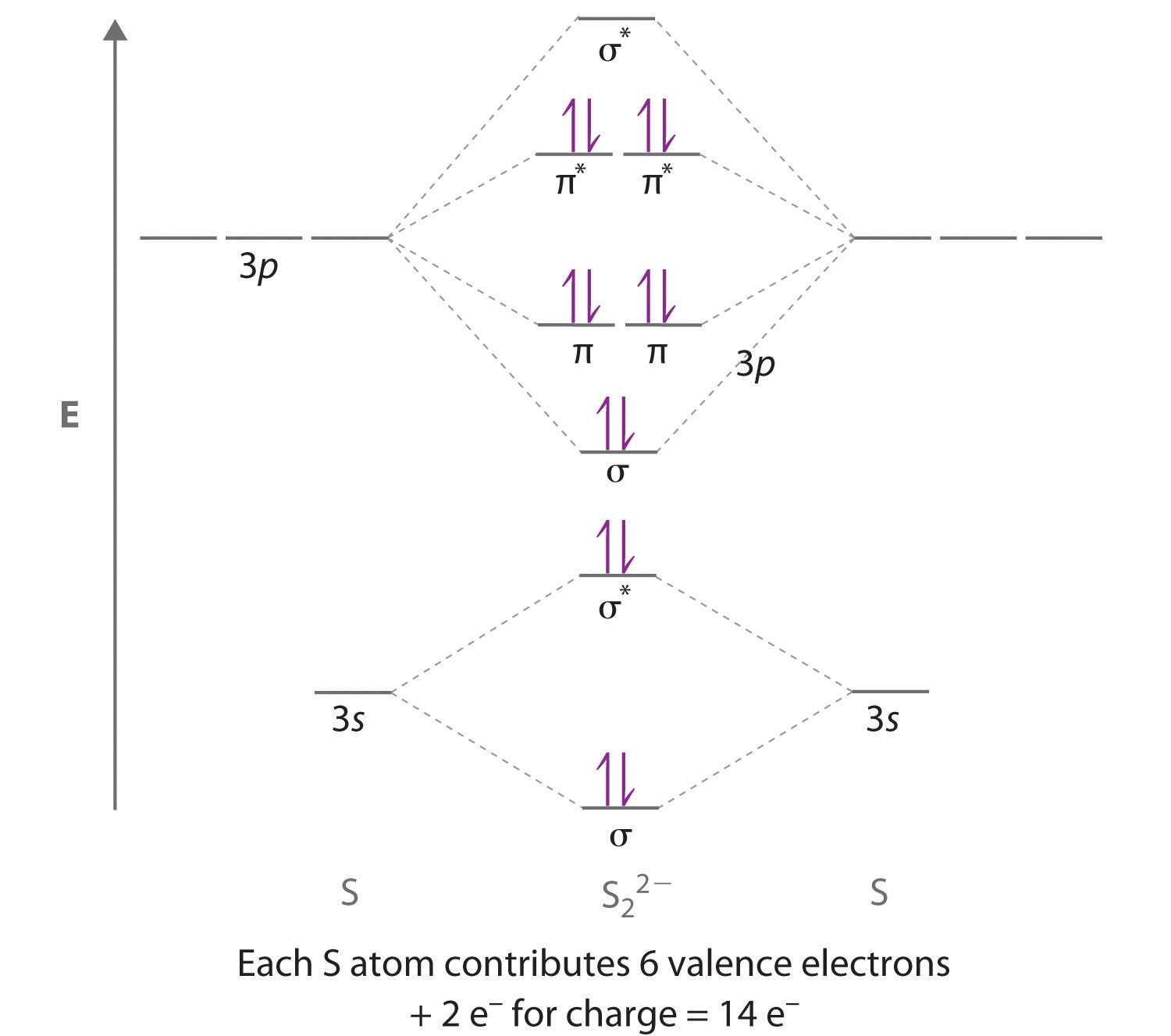 Delocalized Bonding And Molecular Orbitals
Delocalized Bonding And Molecular Orbitals
 Unique C2 Molecular Orbital Diagram 8 4 Theory Chemistry
Unique C2 Molecular Orbital Diagram 8 4 Theory Chemistry
A Brief Introduction To Molecular Orbital Theory Of Simple
 A 1 Molecular Orbital Theory Chemistry Libretexts
A 1 Molecular Orbital Theory Chemistry Libretexts
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Molecular Orbital Energy Level Diagram For N2 A Is Shown That Has An
0 Response to "B2 2 Molecular Orbital Diagram"
Post a Comment