Use The Mo Diagram Given To Find The Bond Order And Predict Whether H2 Exists
Bond order is of bonding electrons antibonding electrons 2. Bond order number of electrons in bonding molecules number of electrons in antibonding molecules2.
 Solution Given The Molecular Orbital Diag Clutch Prep
Solution Given The Molecular Orbital Diag Clutch Prep
Answer to use the mo diagram given to find the bond order and predict whether h2 exists.

Use the mo diagram given to find the bond order and predict whether h2 exists. 8 2 2 62 3. Hybridization is a concept which is quite useful in explaining the shape of molecules. A the bond order of n2 b whether n2 is paramagnetic or diamagnetic c order of increasing bond strength for n2 n22 n2.
In molecular orbital theory bond order is also defined as half of the difference between the number of bonding and antibonding electrons. Bo 12bonding electrons antibonding electrons 122 2 1 1 2 2 122 1 that is we expect boron to form this compound with itself. The repulsion of a lone pair of electrons for another lone pair is greater than that between a bond and a lone pair which in turn is stronger than that between two bond pairs.
According to this two or more than thw non equivalent orbitals with. For a straightforward answer. Use molecular orbital theory to determine.
In this case there are 8 bonding electrons and 2 antibonding. Enter the bond order as a decimal number. Sigma 2s2 sigma 2s2 pi 2p4 sigma 2p1.
According to mot number of atomic orbitals combined is equal to total number of molecular orbitals formedelectronic configuration of h is 1s1. Use an mo diagram to find the bond order and predict whether h2 exists. H22 the mo scheme is σ σ with a bond order of 0 unbound.
You may want to reference pages 371 382section. For the species n2 is diamagnetic because it has no unpaired electrons. The following is part of a molecular orbital energ.
In order to predict the bond order molecular orbital diagram for h2 is to be drawn. So the bond order is. Using the mo diagram predict the bond order for t.
B for n2 the mo diagram is. Home study science chemistry chemistry questions and answers use an mo diagram to find the bond order and predict whether h2 exists. More advanced mo theory shows that σ is more destabilized with respect to the constituent 1s aos than the σ is stabilized so the σ 2e σ 2e configuration is at a higher energy a repulsive interaction than two separated he atoms.
However since you asked for b2 we remove one bonding pi2py electron decreasing the bond order by 12. The bond order for b2 would be.
 Solved Consider The H2 Ion A Sketch The Molecular Orbitals
Solved Consider The H2 Ion A Sketch The Molecular Orbitals
 Energy Level Diagram For Molecular Orbitals Chemical Bonding And
Energy Level Diagram For Molecular Orbitals Chemical Bonding And
 Use An Mo Diagram To Find The Bond Order And Predict Whether H2
Use An Mo Diagram To Find The Bond Order And Predict Whether H2
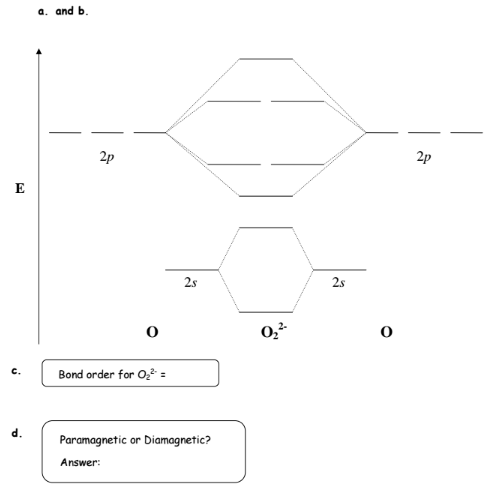 Solution Complete The Mo Energy Level Dia Clutch Prep
Solution Complete The Mo Energy Level Dia Clutch Prep

 Introduction To Molecular Orbital Theory
Introduction To Molecular Orbital Theory

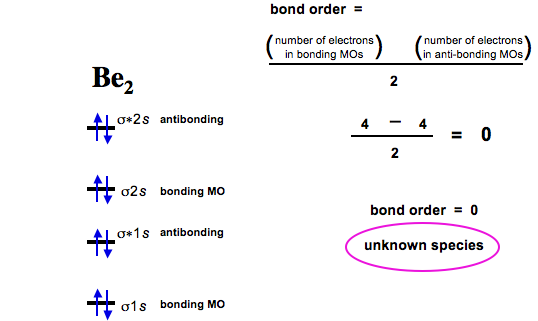 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 3 Ways To Calculate Bond Order In Chemistry Wikihow
3 Ways To Calculate Bond Order In Chemistry Wikihow
 Free Diagram For Student Molecular Orbital Diagram For H2 And Bond
Free Diagram For Student Molecular Orbital Diagram For H2 And Bond

 Spring 2019 1 Determine The Bond Order For The F Chegg Com
Spring 2019 1 Determine The Bond Order For The F Chegg Com
 Molecular Orbital Theory Predicting The Stability Of A Molecule
Molecular Orbital Theory Predicting The Stability Of A Molecule
 Write Molecular Orbital Configuration Of C2 Predict Magnetic
Write Molecular Orbital Configuration Of C2 Predict Magnetic
 According To The Molecular Orbital Theory What Is The Bond Order In
According To The Molecular Orbital Theory What Is The Bond Order In
 What Is The Bond Order Of He2 A 0 B C 1 D
What Is The Bond Order Of He2 A 0 B C 1 D
 Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
 Molecular Orbital Theory Predicting The Stability Of A Molecule
Molecular Orbital Theory Predicting The Stability Of A Molecule
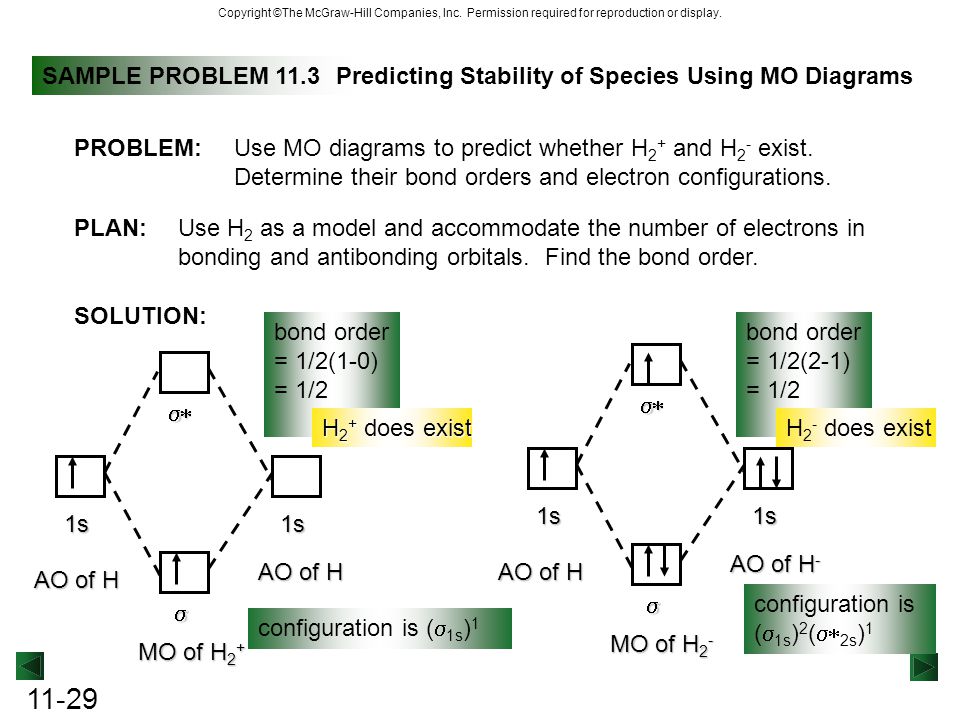 Chapter 11 Theories Of Covalent Bonding Ppt Video Online Download
Chapter 11 Theories Of Covalent Bonding Ppt Video Online Download
 Introducing Ddec6 Atomic Population Analysis Part 3 Comprehensive
Introducing Ddec6 Atomic Population Analysis Part 3 Comprehensive
 Chapter 11 Theories Of Covalent Bonding Ppt Video Online Download
Chapter 11 Theories Of Covalent Bonding Ppt Video Online Download

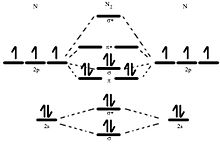 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 3 Ways To Calculate Bond Order In Chemistry Wikihow
3 Ways To Calculate Bond Order In Chemistry Wikihow
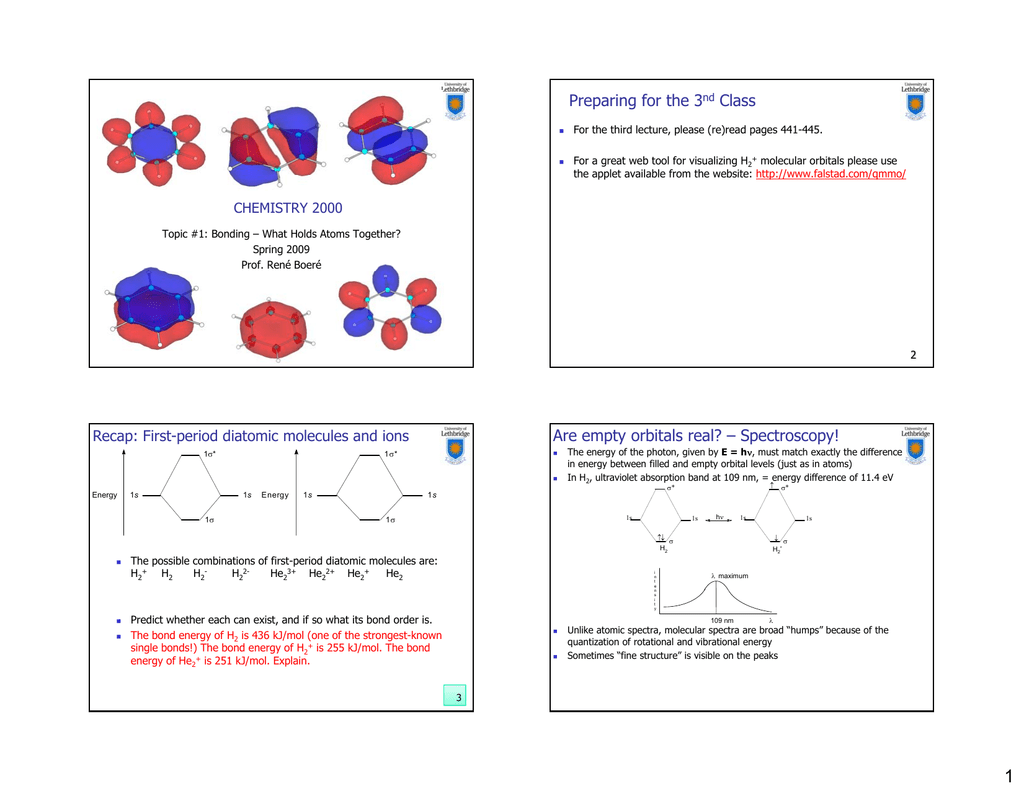
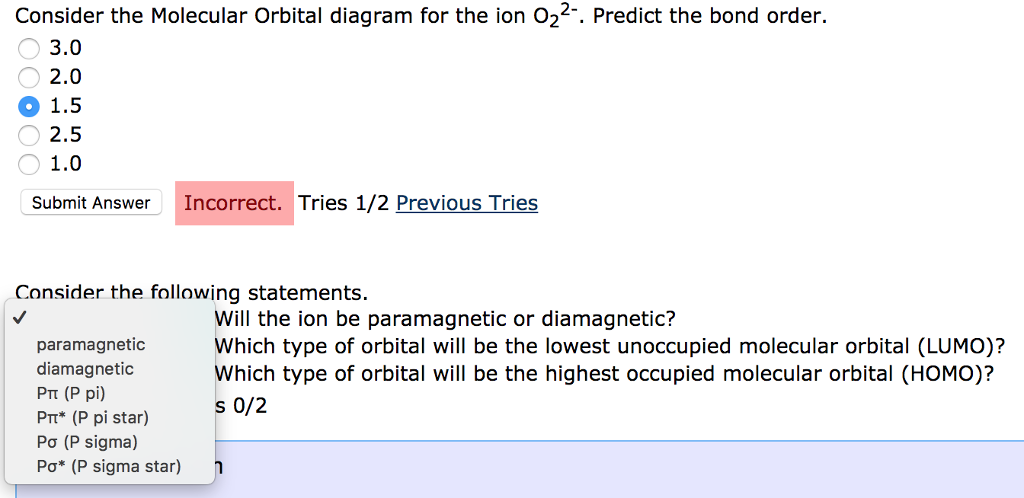
0 Response to "Use The Mo Diagram Given To Find The Bond Order And Predict Whether H2 Exists"
Post a Comment