Use The Orbital Diagram For Oxygen To Write Quantum Numbers For The 4th Electron Of The O Atom
List all of the orbital types subshells in order from least energy to most energy. Also for simplicity each electron has the same n and l quantum numbers of 2 and 1.
 How Are Electrons Distributed In Different Orbits Electronic
How Are Electrons Distributed In Different Orbits Electronic
The first number is the principal quantum number n and the letter represents the value of l angular momentum quantum number.
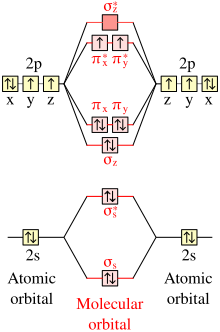
Use the orbital diagram for oxygen to write quantum numbers for the 4th electron of the o atom. There are four different classes of electron orbitals. Relevant equations pauli exclusion principle. Orbital diagrams use the same basic format but instead of numbers for the electrons they use and arrows as well as giving each orbital its own line to represent the spins of the electrons too.
1s2 2s2 2p6 3s2 3p4. The diagram shows the number of subshell by using boxes or lines for electrons use three for p orbitals five for d orbitals and 7 for f orbitals. In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron.
N4 l0 ml0 ms12. Note that the last term in the oxygen electron configuration will be 1s2 2s2 2p4. Write down the quantum numbers itexnlmlmsitex for each one of the electrons.
N represents the energy level l is associated with the sublevel ml represents the orbital and ms is the electron spin. The numbers in order represent energy level type of orbital which orbital which electron. I have trouble for the electrons on the 2p shell.
What are the four quantum numbers for each of the two electrons in a 4s orbital. Write the orbital diagram for sulfur and determine its number of unpaired electrons. The attempt at a solution itex1s22s22p4itex.
In this video well use the electron configuration chart to help us write the notation for oxygen. Use the periodic table. What are the four quantum numbers for each electron in a oxygen atom.
1 s 2 p 3 d and 4 f for the orbital and the superscript number tells you how many electrons are in that orbital. An orbital is a wave function for an electron defined by the three quantum numbers n ℓ and m ℓ. Least energy principle for filling the shells.
10012 energy level 1 0 s orbital type 0 which s orbital theres only one. Write down the electonic configuration for the o atom. These orbitals are determined by the value of the angular momentum quantum number ℓ.
Orbitals define regions in space where you are likely to find electrons. It discusses the 4 quantum numbers n l ml and ms. Chemistry chapter 4 and 5.
 S P D F Orbitals Explained 4 Quantum Numbers Electron
S P D F Orbitals Explained 4 Quantum Numbers Electron
 Electron Configurations Walkthrough Periodic Table Video
Electron Configurations Walkthrough Periodic Table Video
 Electron Configuration For Sulfur S
Electron Configuration For Sulfur S
 Azimuthal Quantum Number Wikipedia
Azimuthal Quantum Number Wikipedia
 Chem4kids Com Oxygen Orbital And Bonding Info
Chem4kids Com Oxygen Orbital And Bonding Info
 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
 Ch150 Chapter 2 Atoms And Periodic Table Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 Chem4kids Com Oxygen Orbital And Bonding Info
Chem4kids Com Oxygen Orbital And Bonding Info
 8 2 Hybrid Atomic Orbitals Chemistry
8 2 Hybrid Atomic Orbitals Chemistry
 Chem4kids Com Oxygen Orbital And Bonding Info
Chem4kids Com Oxygen Orbital And Bonding Info
 Molecular Orbital Theory Chemistry For Majors
Molecular Orbital Theory Chemistry For Majors
 High School Chemistry Electron Configurations Of Main Group Elements
High School Chemistry Electron Configurations Of Main Group Elements
 Electron Shells Orbitals The Periodic Table Article Khan Academy
Electron Shells Orbitals The Periodic Table Article Khan Academy
 Electron Configuration And Orbital Diagrams Ppt Video Online Download
Electron Configuration And Orbital Diagrams Ppt Video Online Download
 Magnetic Quantum Number Definition Example Video Lesson
Magnetic Quantum Number Definition Example Video Lesson
 Electronic Structure Of Atoms Introductory Chemistry Lecture Lab
Electronic Structure Of Atoms Introductory Chemistry Lecture Lab
 Electron Configuration Chemistry Libretexts
Electron Configuration Chemistry Libretexts
 Quantum Numbers And Electron Configurations
Quantum Numbers And Electron Configurations
 Electron Configurations In The 3d Orbitals Video Khan Academy
Electron Configurations In The 3d Orbitals Video Khan Academy
 Pauli Exclusion Principle Chemistry Libretexts
Pauli Exclusion Principle Chemistry Libretexts
 Ch150 Chapter 2 Atoms And Periodic Table Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 Electron Configurations The Periodic Table
Electron Configurations The Periodic Table
 Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
 Electronic Structure Of Atoms Introductory Chemistry Lecture Lab
Electronic Structure Of Atoms Introductory Chemistry Lecture Lab
 Chapter 3 Atomic Structure And Properties
Chapter 3 Atomic Structure And Properties
 Electronic Configurations Using Arrows Youtube
Electronic Configurations Using Arrows Youtube
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Spin Quantum Number Definition Example Video Lesson
Spin Quantum Number Definition Example Video Lesson
 Organization Of Electrons In Atoms Introductory Chemistry 1st
Organization Of Electrons In Atoms Introductory Chemistry 1st

0 Response to "Use The Orbital Diagram For Oxygen To Write Quantum Numbers For The 4th Electron Of The O Atom"
Post a Comment