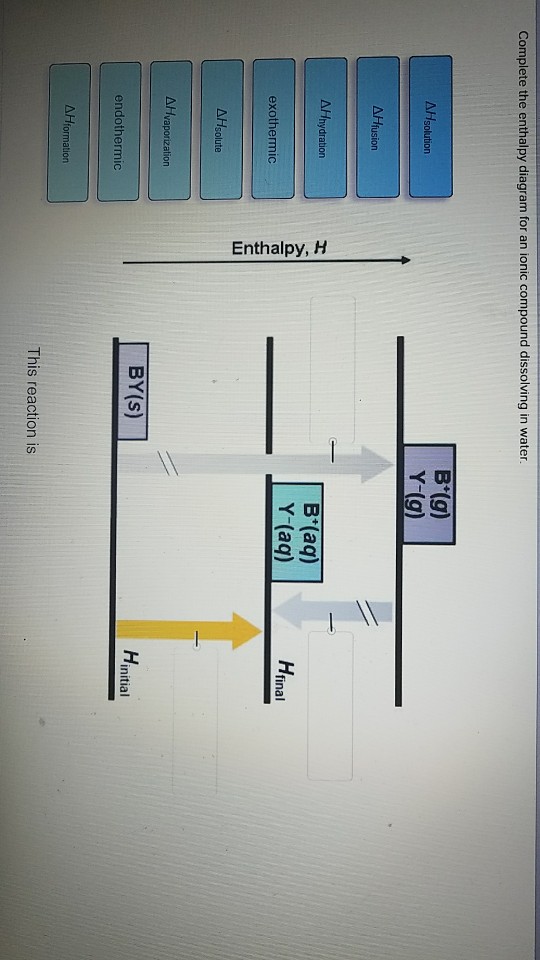
Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water
When understanding the enthalpy of solution it is easiest to think of a hypothetical three. Yg to determine the process of dissolving 1onic compounds in water is endothermic or exothermic first calculate the enthalpy of solution as follows ahsolute baq y aq the enthalpy of solutionhoattice ener.
Ch150 Chapter 7 Solutions Chemistry
Whether the dissolution process of a given ionic compound gives off or absorbs heat depends on the strength of the intermolecular forces holding the solid together as well as those between the ions and the water once dissolved.
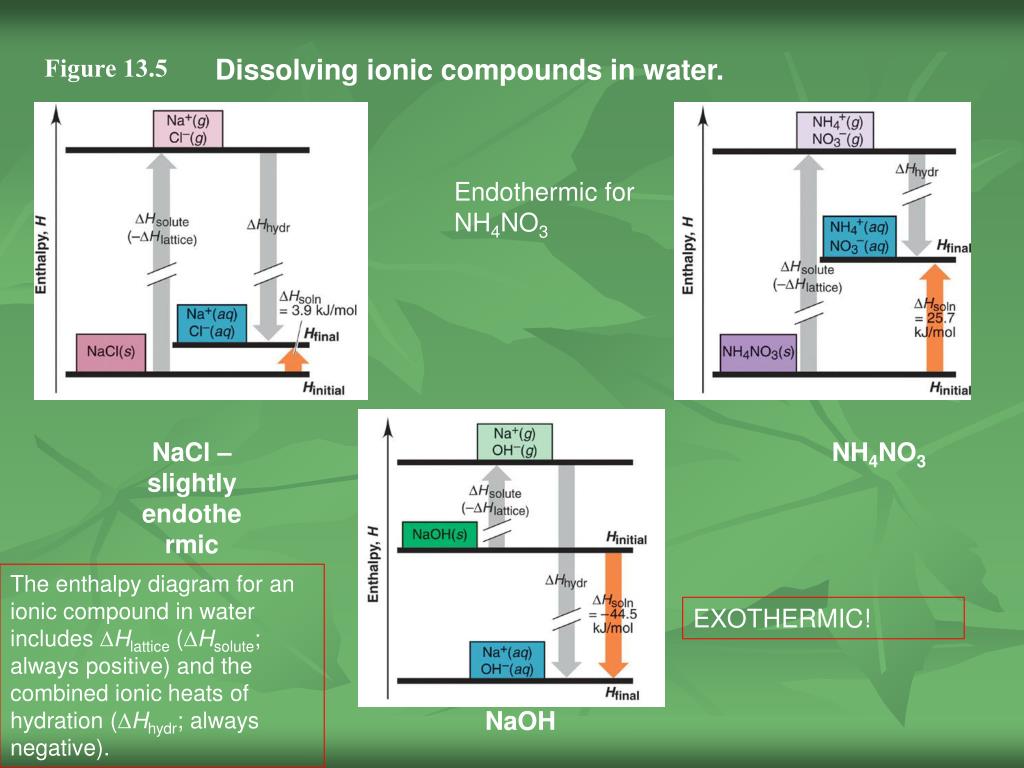
Complete the enthalpy diagram for an ionic compound dissolving in water. With the aid of a diagram show the enthalpy changes involved in the dissolving of an ionic compound in water where enthalpy change of solution is endothermic. Complete the enthalpy diagram for an ionic compound dissolving in water b g y g ahsolution b. This enthalpy of solution δhsolution can either be positive endothermic or negative exothermic.
With the aid of a diagram show the enthalpy changes involved in the dissolving of an ionic compound in water where enthalpy change of solution is exothermic. The strength of the ionic attractions in the lattice is measured using the lattice energy. Get 11 help now from expert chemistry tutors.
Endothermic delta h fusion delta h formation delta h solute delta h hydration exothermic delta h vaporization delta h solution this reaction is. This energy tends to stop substances dissolving unless the energy is paid back in later. This lesson occurs at the end of this unit at a point where students are already expected to understand ionic bonds ions and polarity.
Complete the enthalpy diagram for an ionic compound dissolving in water. Get more help from chegg. Using the dissolution of ionic compounds in water to explore thermodynamics.
The lattice energy must be supplied in order to break down a lattice and enable the ionic substance to dissolve in water. In this exercise you will examine the dissolution of various salts in water. Students are drawing a model of an ionic compound dissolving in water sep 2 and providing explanations for how the dissolution occurs sep 6.
The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process at constant pressure.
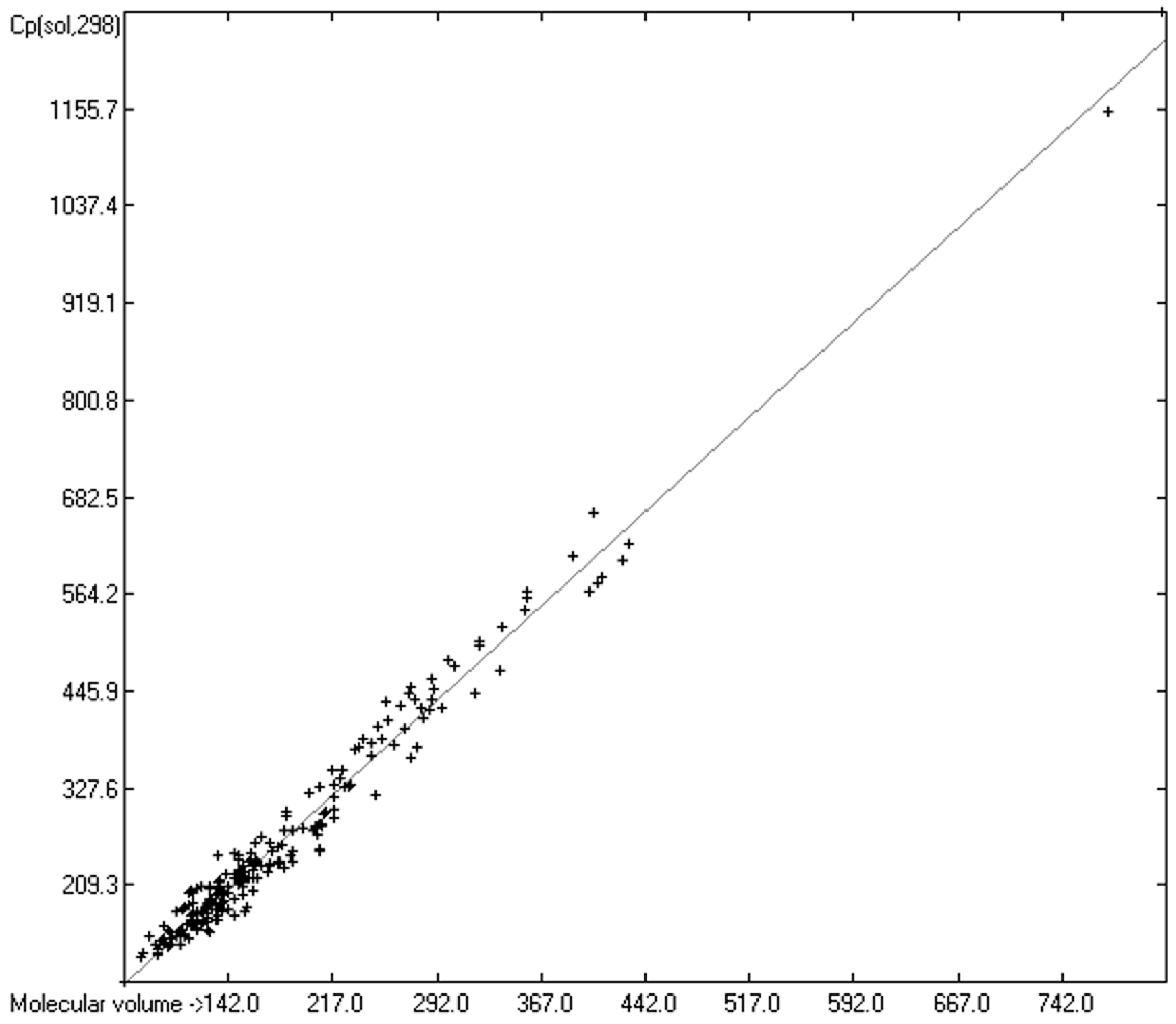 Molecules Free Full Text Calculation Of The Isobaric Heat
Molecules Free Full Text Calculation Of The Isobaric Heat
5 7 Naming Ionic Compounds Chemistry Libretexts
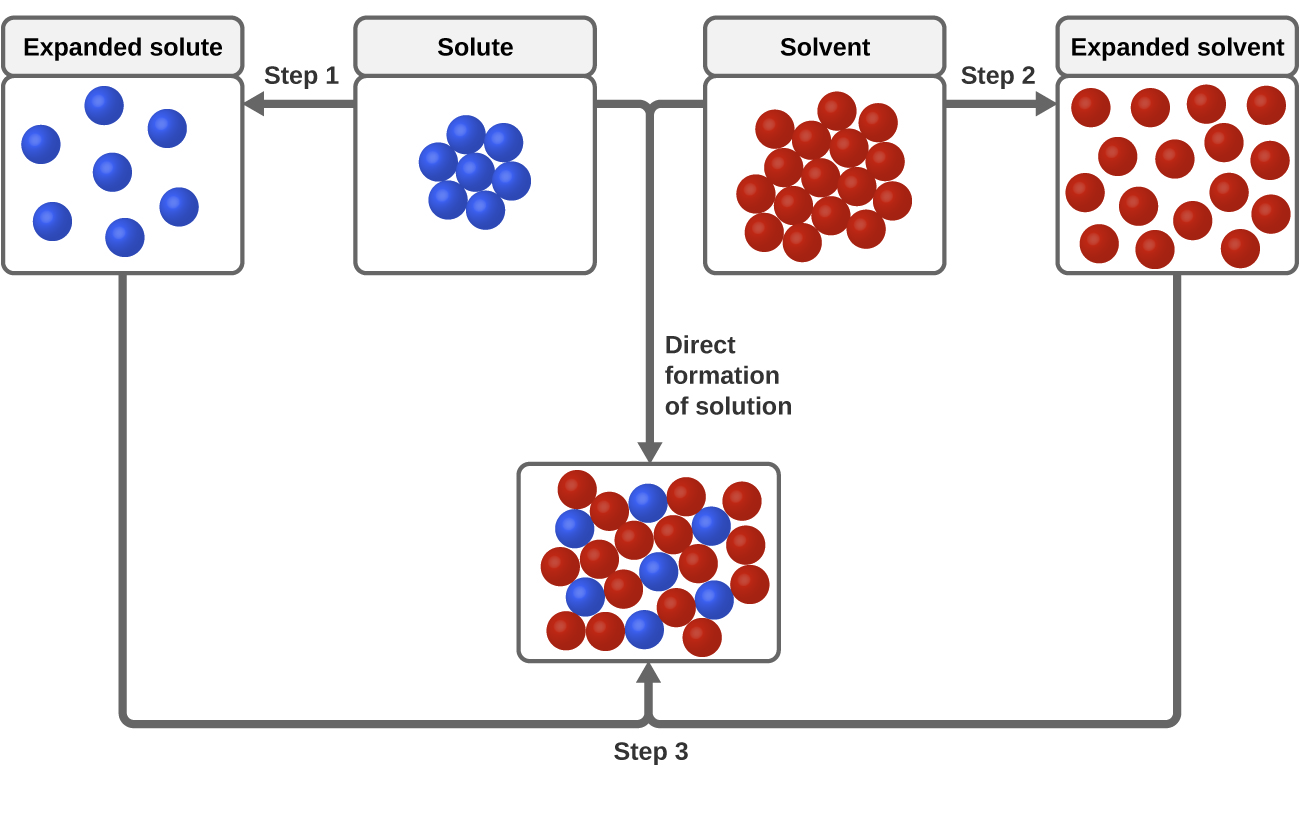 11 1 The Dissolution Process Chemistry
11 1 The Dissolution Process Chemistry
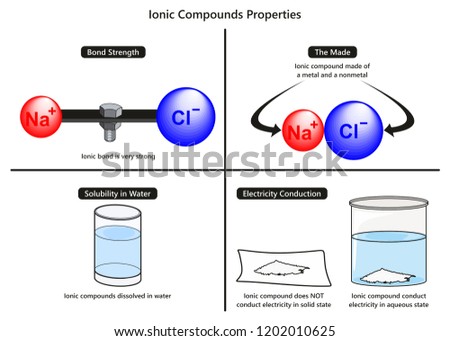 Ionic Bond Properties Infographic Diagram Including Stock
Ionic Bond Properties Infographic Diagram Including Stock
 Internal Energy And Enthalpy Springerlink
Internal Energy And Enthalpy Springerlink
 Solution Chemistry Grandinetti Group
Solution Chemistry Grandinetti Group
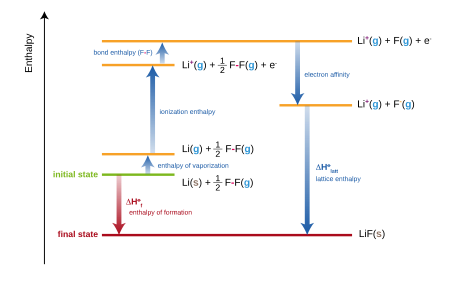
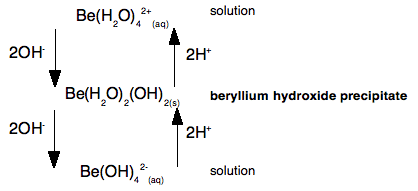 Chemistry Of Beryllium Untypical Of Group 2
Chemistry Of Beryllium Untypical Of Group 2
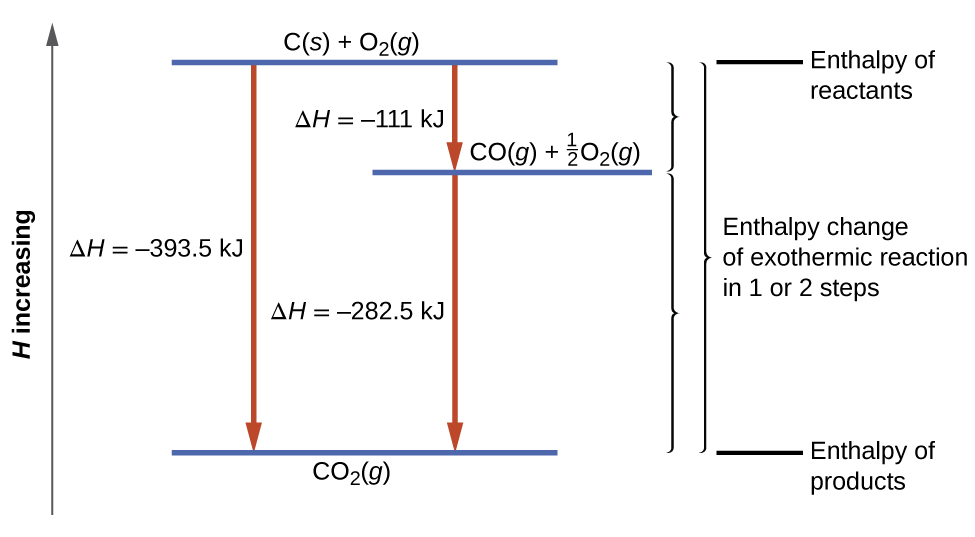
A Level The Hess S Law Cycle Calculation Of The Energy Changes When
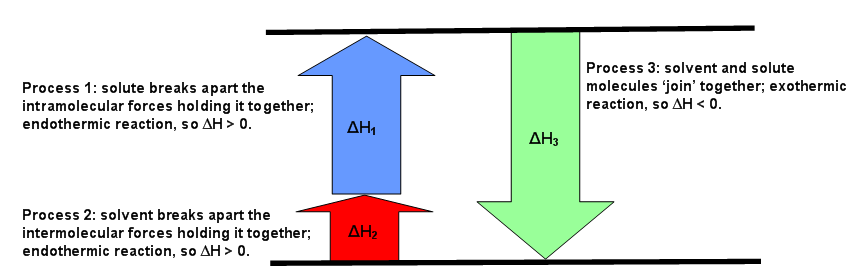 Enthalpy Of Solution Chemistry Libretexts
Enthalpy Of Solution Chemistry Libretexts
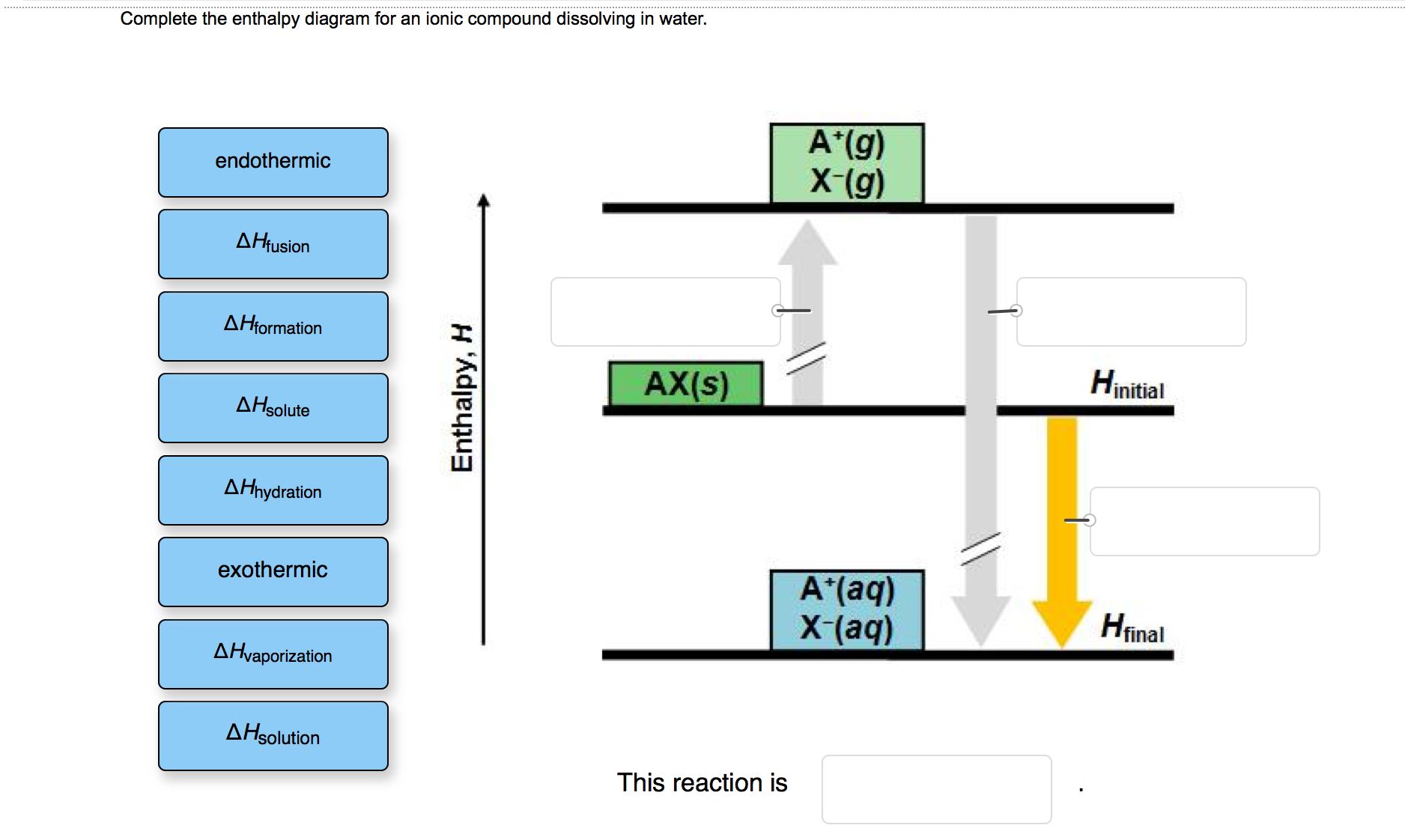 Solved Which Box On The Left Fits Into Which Space On The
Solved Which Box On The Left Fits Into Which Space On The
 Ppt The Properties Of Solutions Powerpoint Presentation Id 3592416
Ppt The Properties Of Solutions Powerpoint Presentation Id 3592416
 The Molecular Nature Of Matter And Change Ppt Download
The Molecular Nature Of Matter And Change Ppt Download

The Chemistry Of The Halogens 2
13 1 Factors Affecting Solution Formation Chemistry Libretexts
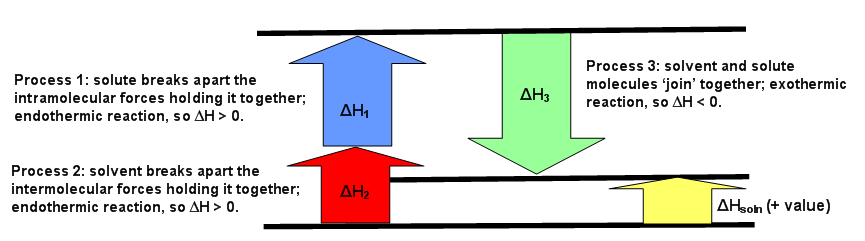 Enthalpy Of Solution Chemistry Libretexts
Enthalpy Of Solution Chemistry Libretexts
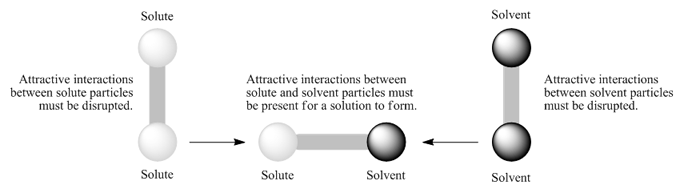 Lab 11 Thermodynamics Of Salt Dissolution
Lab 11 Thermodynamics Of Salt Dissolution
 The Ionic Bond Boundless Chemistry
The Ionic Bond Boundless Chemistry
 The Molecular Nature Of Matter And Change Ppt Download
The Molecular Nature Of Matter And Change Ppt Download

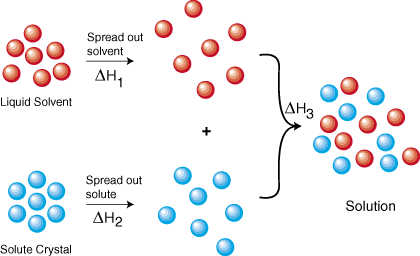

0 Response to "Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water"
Post a Comment