Orbital Filling Diagram For Silicon
The nex six electrons will go in the 2p orbital. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.
 Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Dublin Schools Lesson Orbital Diagrams And Electron Configurations
The outer most electrons are the only ones included in the orbital filling diagram and the electron dot diagram because the outer most electrons are the only ones that need to be used in chemical.
Orbital filling diagram for silicon. So you put 8 electrons into your energy level diagram. You can represent electrons as arrows. Also the crystalline form is used in semiconductors.
If you havent yet learned electron configurations you really need to go ahead. Commercial production depends on a reaction between sand sio2 and carbon at a. The first electron goes into the 1s orbital filling the lowest energy level first and the second one spin pairs with the first one.
Now that youve mastered the world of electron configurations its time to write orbital filling diagrams. Electron configuration of silicon si orbital diagram. Silicon carbide sic is one of the hardest substances known and used in polishing.
Electron configuration of silicon si orbital diagram and noble gas configuration. This sounds like something that would be tough but orbital filling diagrams are really just pictures that show you the same thing as electron configurations. Sources makes up major portion of clay granite quartz sio2 and sand.
Well put six in the 2p orbital and then put the next two. If two electrons end up in the same orbital one arrow faces up and the other faces down. Visit the post for more.
The p orbital can hold up to six electrons. In an orbital filling diagram the individual orbitals are shown as circles or squares and orbitals within a sublevel are drawn next to each other horizontally. Since 1s can only hold two electrons the next 2 electrons for silicon go in the 2s orbital.
Although drawing out each orbital may prove to be helpful in determining unpaired electrons it is very time consuming and often not this figure includes electron configurations and orbital diagrams for four elements n o correctly following hund s rule will have an effect on the number of unpaired electrons oxygen orbital filling diagram serves as example 3 example draw. In writing the electron configuration for silicon the first two electrons will go in the 1s orbital. How to write electron configurations and orbital diagrams.
Orbital diagram electron configuration and the noble gas notation for a silicon si atom.
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Solved Fill In The Orbital Energy Diagram For The Nitroge
Solved Fill In The Orbital Energy Diagram For The Nitroge
 Latest Of Orbital Filling Diagram For Bromine File Electron
Latest Of Orbital Filling Diagram For Bromine File Electron
 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
 How Many Bonding Orbitals Are In Silicon Tetraflouride Or Sif 4
How Many Bonding Orbitals Are In Silicon Tetraflouride Or Sif 4
 Spin And Orbital Structure Of The First Six Holes In A Silicon Metal
Spin And Orbital Structure Of The First Six Holes In A Silicon Metal
 L 650 Nm 6 50 X 10 7 M N C X 108 M S 4 61 X 1014 Hz Ppt
L 650 Nm 6 50 X 10 7 M N C X 108 M S 4 61 X 1014 Hz Ppt
 Crystal Orbital Overlap Population Coop Diagrams The Dotted
Crystal Orbital Overlap Population Coop Diagrams The Dotted
 Mo Diagrams For Diatomic Molecules
Mo Diagrams For Diatomic Molecules
 The 1s Orbital Is Filled First And When It Is Filled We Have An
The 1s Orbital Is Filled First And When It Is Filled We Have An
 8 3 Electron Configurations How Electrons Occupy Orbitals
8 3 Electron Configurations How Electrons Occupy Orbitals
 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
 The Order Of Filling 3d And 4s Orbitals
The Order Of Filling 3d And 4s Orbitals
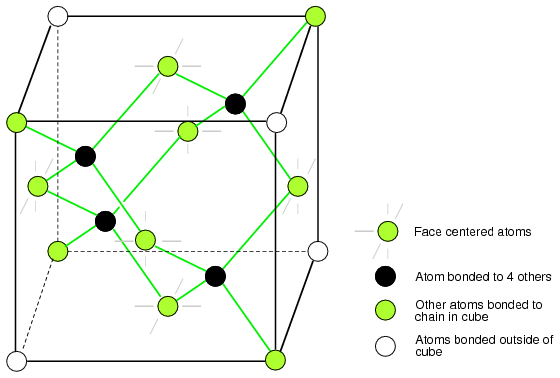
 Valence And Crystal Structure Solid State Device Theory
Valence And Crystal Structure Solid State Device Theory
 11 7 Bonding In Metals Chemistry Libretexts
11 7 Bonding In Metals Chemistry Libretexts
 Quantum Mechanics Quantum Mechanics Orbitals In Quantum Mechanics
Quantum Mechanics Quantum Mechanics Orbitals In Quantum Mechanics
 What Is The Orbital Diagram Of Calcium How Was It Originally
What Is The Orbital Diagram Of Calcium How Was It Originally
 Hyperconjugation Mechanism Mordor
Hyperconjugation Mechanism Mordor
 Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its
Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its
 A Linear Cobalt Ii Complex With Maximal Orbital Angular Momentum
A Linear Cobalt Ii Complex With Maximal Orbital Angular Momentum
 Electron Configuration For Silicon Si
Electron Configuration For Silicon Si


0 Response to "Orbital Filling Diagram For Silicon"
Post a Comment