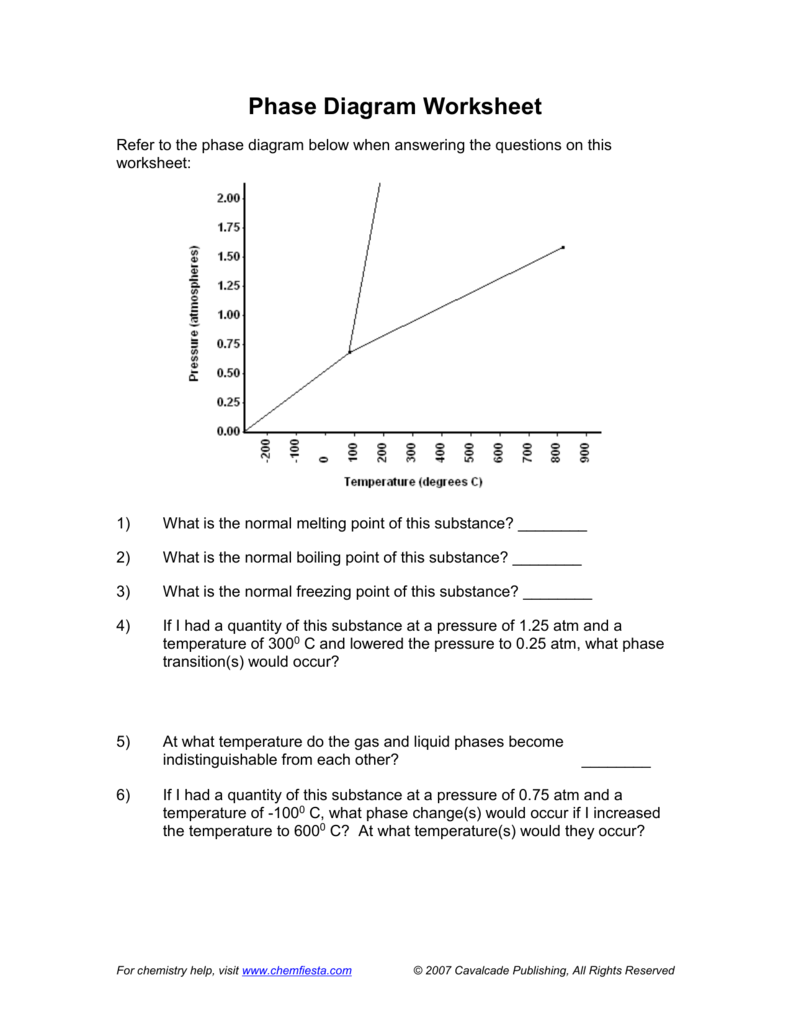
The Normal Boiling Point For The Substance In The Phase Diagram Below Is Approximately
For the mp id read approximately 180k and for the nbp about 300k. C what is the physical state of the substance under t 150 k p 02 atm.
 Ap Chemistry Problem Set Chapter 10
Ap Chemistry Problem Set Chapter 10
Express your answer using two significant figures.

The normal boiling point for the substance in the phase diagram below is approximately. A triple point at 5 atm and 5c. Imagine a substance with the following points on the phase diagram. Point c is the critical point of the substance which is the highest temperature and pressure at which a gas and a liquid can coexist at equilibrium.
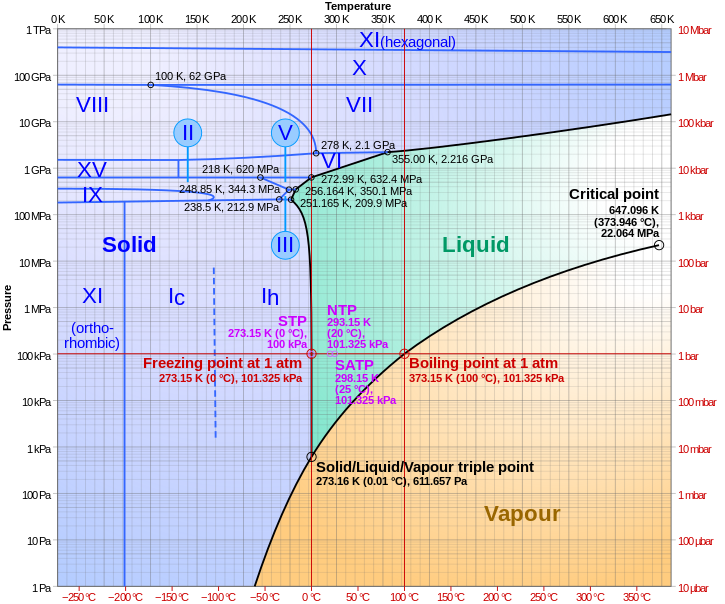
In the generic phase diagram shown below you can see three curves that separate phases colour coded space on the diagram. Ii iii only c. The normal boiling point is just one point where the vapourliquid equilibrium line occurs at one atmosphere pressure.
Express your answer using two significant figures. O normal boiling point condensation point the temperature at which the vapor pressure of a liquid is equal to standard pressure 100 atm 760 mmhg 760 torr 101325 kpa phase diagram for water for water the liquid phase is more dense than the solid phase due to hydrogen bonding. Figure 1 a estimate the normal boiling point of the substance.
A approximately what is the normal boiling point and what is the normal melting point of the substance. A normal melting point at 20c. Which explains why a single step mechanism might result a slow rate for this reaction.
It is therefore called the triple point of the substance and it represents the only point in the phase diagram in which all three states are in equilibrium. If you can read graphs this should be easy. Another scientists suggests the reaction 2 ce4 aq tl aq 2 ce3 aq tl3 aq proceeds via a single elementary step rather than the mechanism above.
And a critical point at 5 atm and 1000c. The phase diagram of a substance is shown below. Which describes the role of the manganese ii ion in this mechanism.
A phase diagram is a lot of points at differing temperatures and pressures. B estimate the normal freezing point of the substance. The phase diagram of a substance is shown below.
The approximate normal boiling point of this substance is 300 k imagine a reaction that results in a change in both volume and temperature as shown in the diagram below. The word normal here refers to 1 atm pressure. A normal boiling point at 150c.
The phase diagram of a hypothetical substance is shown below. Approximately what is the normal boiling point.
 Triple Point An Overview Sciencedirect Topics
Triple Point An Overview Sciencedirect Topics
 Consider The Phase Diagram Shown Choose The Stateme
Consider The Phase Diagram Shown Choose The Stateme
 Phase Diagrams Of Water Co2 Explained Chemistry Melting
Phase Diagrams Of Water Co2 Explained Chemistry Melting
 Phase Diagrams Critical Point Triple Point And Phase Equilibrium
Phase Diagrams Critical Point Triple Point And Phase Equilibrium
 Boiling Point From Pvt Diagram Example Youtube
Boiling Point From Pvt Diagram Example Youtube
Solved The Phase Diagram Of A Substance Is Shown Below A
Phase Change Diagram Boiling Point Co2 Of And The Temperature
 What Is Triple Point Phase Diagram Of Water Mechanical
What Is Triple Point Phase Diagram Of Water Mechanical
 Intermolecular Forces Interpreting A Phase Diagram Chemistry
Intermolecular Forces Interpreting A Phase Diagram Chemistry
 Solved The Phase Diagram Of A Substance Is Shown Below A App
Solved The Phase Diagram Of A Substance Is Shown Below A App
 Lesson 33 Energy And Phase Change Objectives The Student Will
Lesson 33 Energy And Phase Change Objectives The Student Will
Chapter 10 Liquids Solids And Phase Changes
 Solved The Normal Boiling Point Of The Substance With The
Solved The Normal Boiling Point Of The Substance With The

Invariant Points And Gibbs Phase Rule
Chemistry The Central Science Chapter 11 Section 6
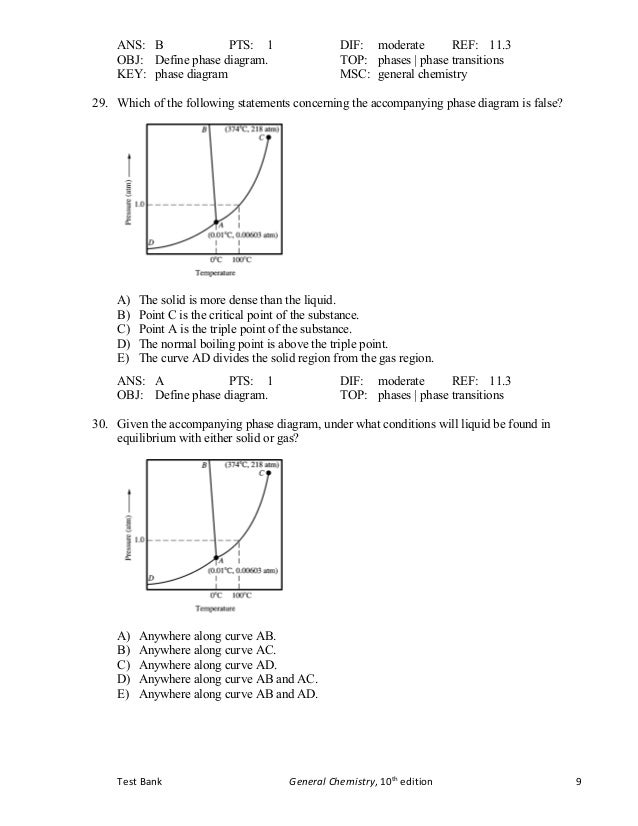 Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
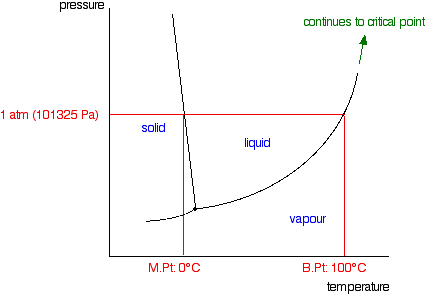 Raoult S Law And Non Volatile Solutes
Raoult S Law And Non Volatile Solutes
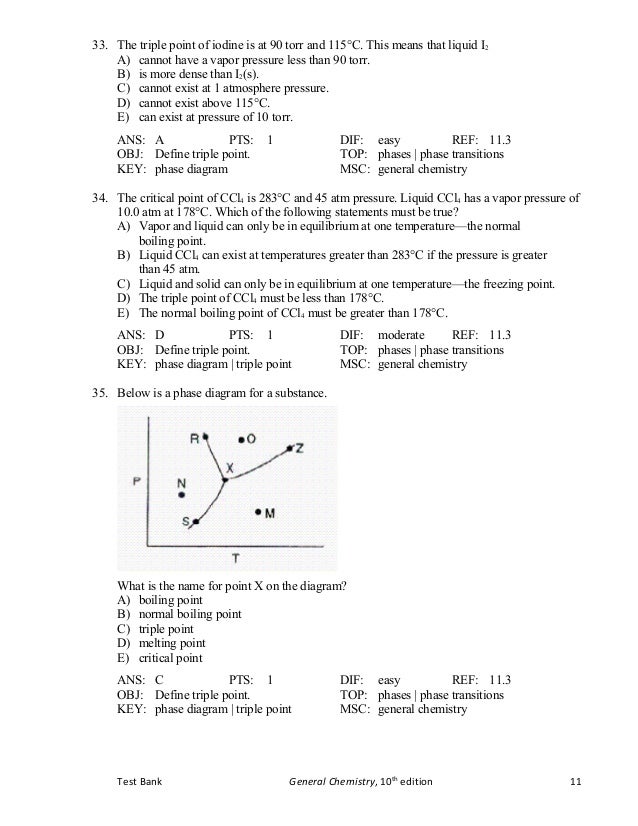 Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
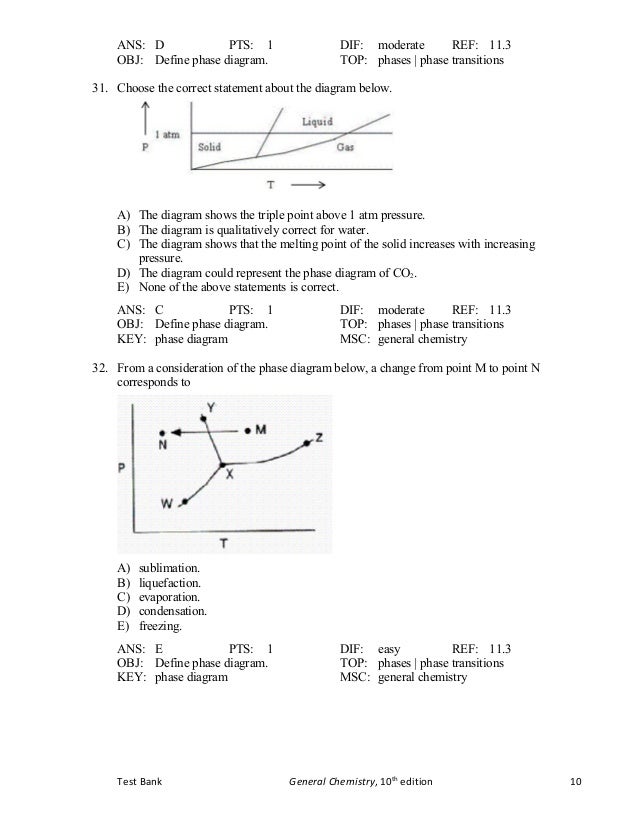 Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
Tb Chapter11 Bbbbbbbbbbbbbbbbbbbbbbbbbb
Of Pressure Vs Temperature Sometimes With The Pressure Axis Scaled
0 Response to "The Normal Boiling Point For The Substance In The Phase Diagram Below Is Approximately"
Post a Comment