How To Calculate Bond Order From Molecular Orbital Diagram
Thus the bond order is two. The bond order describes the stability of the bondthe molecular orbital provides an easy understanding of the concept of the bond order of a chemical bond.
What Is The Bond Order For N2 Quora
Consistent with oxygens double bond.
How to calculate bond order from molecular orbital diagram. We can calculate the bond order in the o 2 molecule by noting that there are eight valence electrons in bonding molecular orbitals and four valence electrons in antibonding molecular orbitals in the electron configuration of this molecule. Do the number of aos number of mos. To obtain the bond order look at the molecular orbitals formed and decide whether they are bonding or antibonding.
H 1s 1 1 valence electron f he 2s 2 2p 5 7 valence electrons step 2. This often but not always yields the same result. How to calculate bond order from molecular orbital diagram molecular orbital mo theory and the bond order.
Bond order number of electrons in bonding molecules number of electrons in antibonding molecules2. In molecular orbital theory we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond. For instance the bond order of diatomic nitrogen nn is 3 and bond order between the carbon atoms in h hc h is also three.
It quantifies the degree of covalent bonds between the atoms. 12 bonding orbitals antibonding orbitals 128 4 2. And this should make sense because no is isoelectronic with co which has a bond order of 3.
For a straightforward answer. Bond order no. In atoms electrons are located on atomic orbitals ao.
Bond order is also an index of bond strength and it is used extensively in valence bond theory. F will be lower on the diagram. Of electrons in anti bonding mo no.
Fill the mos with electrons. Of electrons in bonding mo 2. In molecular orbital diagram we just need to calculate the number of electrons in anti bonding orbital and bonding orbital then we can use the formula in order to calculate bond order is.
In molecular orbital theory bond order is also defined as the difference divided by two between the number of bonding and antibonding electrons. With one additional electron in an antibonding orbital 2b2 the bond order decreases by 1 2 relative to no. If so calculate the bond order.
In molecular orbital theory bond order is also defined as half of the difference between the number of bonding and antibonding electrons.
 3 Ways To Calculate Bond Order In Chemistry Wikihow
3 Ways To Calculate Bond Order In Chemistry Wikihow
 What Is The Bond Order Of Co Quora
What Is The Bond Order Of Co Quora
 Molecular Orbital Theory Boundless Chemistry
Molecular Orbital Theory Boundless Chemistry
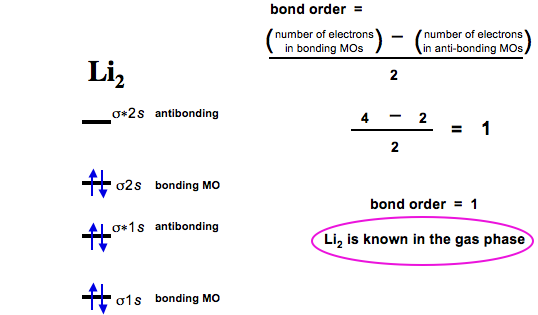 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 How Do I Calculate The Bond Order For H2 And H2 Socratic
How Do I Calculate The Bond Order For H2 And H2 Socratic
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
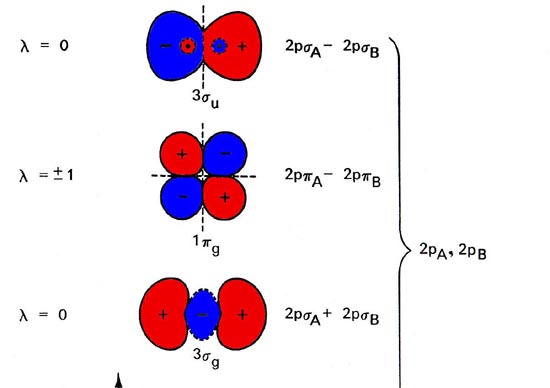 Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics
 Molecular Orbital A Molecule In Which All The Electrons Are Paired
Molecular Orbital A Molecule In Which All The Electrons Are Paired
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
What Charge Would Be Needed On F2 To Generate An Ion With A Bond
 How To Build Molecular Orbitals Chemistry Libretexts
How To Build Molecular Orbitals Chemistry Libretexts
 Multiple Bonds Bond Energies Lengths Molecular Orbital Theory
Multiple Bonds Bond Energies Lengths Molecular Orbital Theory
 Mo Diagram For No Wiring Diagram Schema
Mo Diagram For No Wiring Diagram Schema
Polyatomic Molecular Orbital Theory
Molecular Orbital Theory Mot Chemistry Study Material
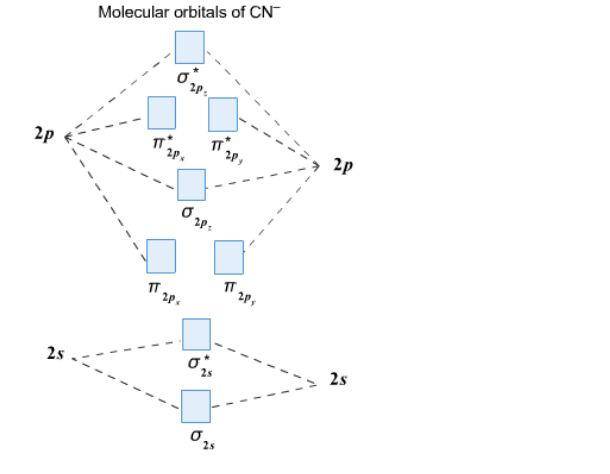 Solution Complete This Molecular Orbital Clutch Prep
Solution Complete This Molecular Orbital Clutch Prep
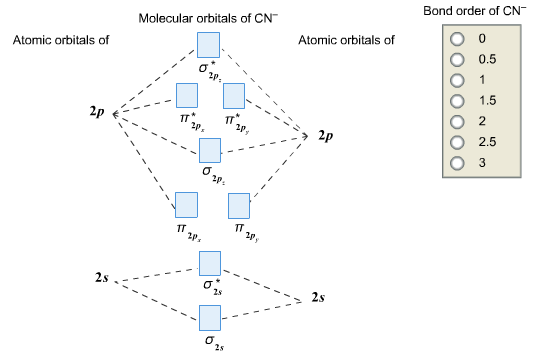 Complete This Molecular Orbital Diagram Fo Clutch Prep
Complete This Molecular Orbital Diagram Fo Clutch Prep
 C2 Molecule Doubly Or Quadruply Bonded Mapping Ignorance
C2 Molecule Doubly Or Quadruply Bonded Mapping Ignorance
 How To Draw Molecular Orbital Diagram 162910 Using The Mo Diagram Of
How To Draw Molecular Orbital Diagram 162910 Using The Mo Diagram Of
Principles Of Chemical Science Solutions For Lecture 13 Molecular
Chem 344 Homework 9 Due Friday Apr 11 2014 2 Pm
 Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
What Is An F2 Bond Order Quora
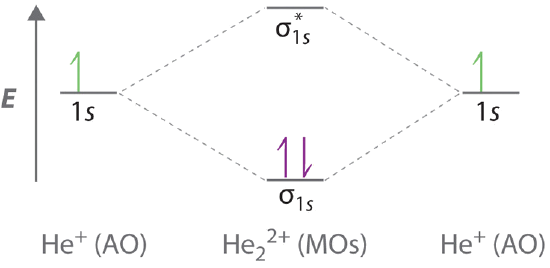 9 8 Molecular Orbital Theory Does Not Predict A Stable Diatomic
9 8 Molecular Orbital Theory Does Not Predict A Stable Diatomic
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order
 Multiple Bonds Bond Energies Lengths Molecular Orbital Theory
Multiple Bonds Bond Energies Lengths Molecular Orbital Theory

0 Response to "How To Calculate Bond Order From Molecular Orbital Diagram"
Post a Comment