What Is The Basis For Exceptions To The Aufbau Diagram
According to the aufbau principle these electrons should always fill shells and subshells according to increasing energy levels. Filled and half filled energy sublevels are more stable than partially filled energy sublevels.
 Chapter 5 Review Electrons In Atoms Ppt Video Online Download
Chapter 5 Review Electrons In Atoms Ppt Video Online Download
Some elements have unusual atomic orbitals read more.

What is the basis for exceptions to the aufbau diagram. What is the basis for exceptions to the aufbau diagram. The latin alphabet forms the basis of the english alphabet it is the same alphabet with the exceptions of j u and w. Despite the exceptions the aufbau principle is useful in chemistry courses where students discover the fundamental rules about the atomic structure and properties of elements.
What is the basis for exceptions to the aufbau principle. You get s orbitals and p orbitals fuzing to create sp. A filled and half filled energy sublevels are more stable than partially filled energy sublevels b electron configurations are only probable.
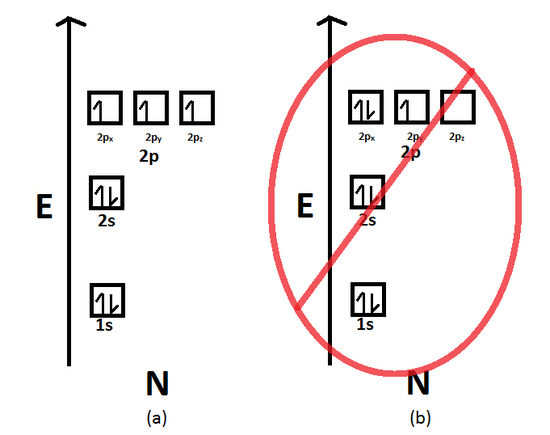
Start studying chemistry chapter 5. This actually reduces electron electron repulsion in the atom since each electron has its own seat in the subshell. What is the basis for exceptions to the aufbau diagram.
The simple answer to your question is just putting unidirectional electrons in the p orbital. Other exceptions are copper and silver. What is the basis for exceptions to the aufbau diagrams.
What are the ratings and certificates for aufbau 1951. Learn vocabulary terms and more with flashcards games and other study tools. Which scientist developed the quantum mechanical model of the atom.
A chart or diagram may be used to show how the principle works for various example elements. But a more complicated way of how the aufbau principle is disregarded is when there is hybridization of the orbitals together. The aufbau principle it states that electrons fill the atomic orbitals of the lowest available energy levels before occupying higher levels.
Elements such as copper and chromium are exceptions because their electrons fill and half fill two subshells with some electrons in the higher energy level shells. When a legal document says on an exceptions basis it means just that. For example the predicted aufbau configuration for cr is 4s 2 3d 4 but the observed configuration is actually 4s 1 3d 5.
 Electron Configurations For The First Period Video Khan Academy
Electron Configurations For The First Period Video Khan Academy
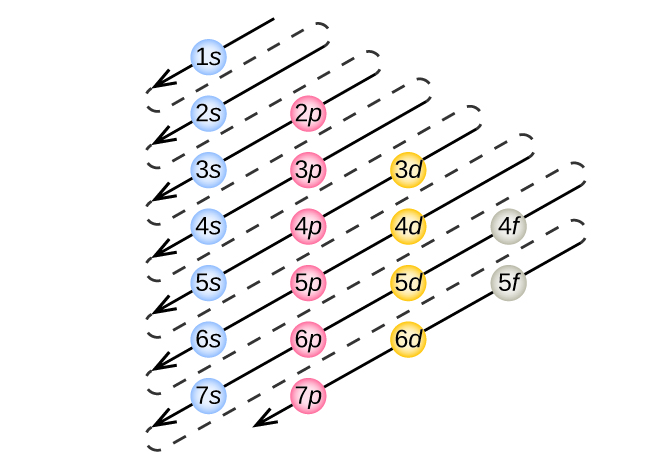 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
 What Is The Electron Configuration Of Ag Socratic
What Is The Electron Configuration Of Ag Socratic
 Electron Configuration For D Block Element Video Khan Academy
Electron Configuration For D Block Element Video Khan Academy
 Electron Configuration Chemistry Libretexts
Electron Configuration Chemistry Libretexts
![]() The Periodic Table Is An Icon But Chemists Still Can T Agree On How
The Periodic Table Is An Icon But Chemists Still Can T Agree On How
 Openstax General Chemistry Ch6 Electronic Structure And Periodic
Openstax General Chemistry Ch6 Electronic Structure And Periodic
 Arrangement Of Electrons In Atoms Ppt Video Online Download
Arrangement Of Electrons In Atoms Ppt Video Online Download
 Ppt Chapter 5 The Periodic Law Powerpoint Presentation Id 5937138
Ppt Chapter 5 The Periodic Law Powerpoint Presentation Id 5937138
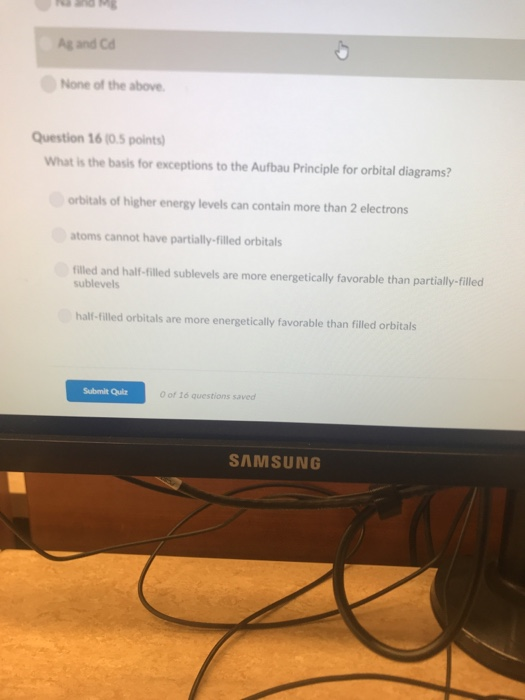 Solved False Question 6 0 5 Point How Many Unpaired Elec
Solved False Question 6 0 5 Point How Many Unpaired Elec
 Multi Electron Atoms And The Periodic Table Book Chapter Iopscience
Multi Electron Atoms And The Periodic Table Book Chapter Iopscience

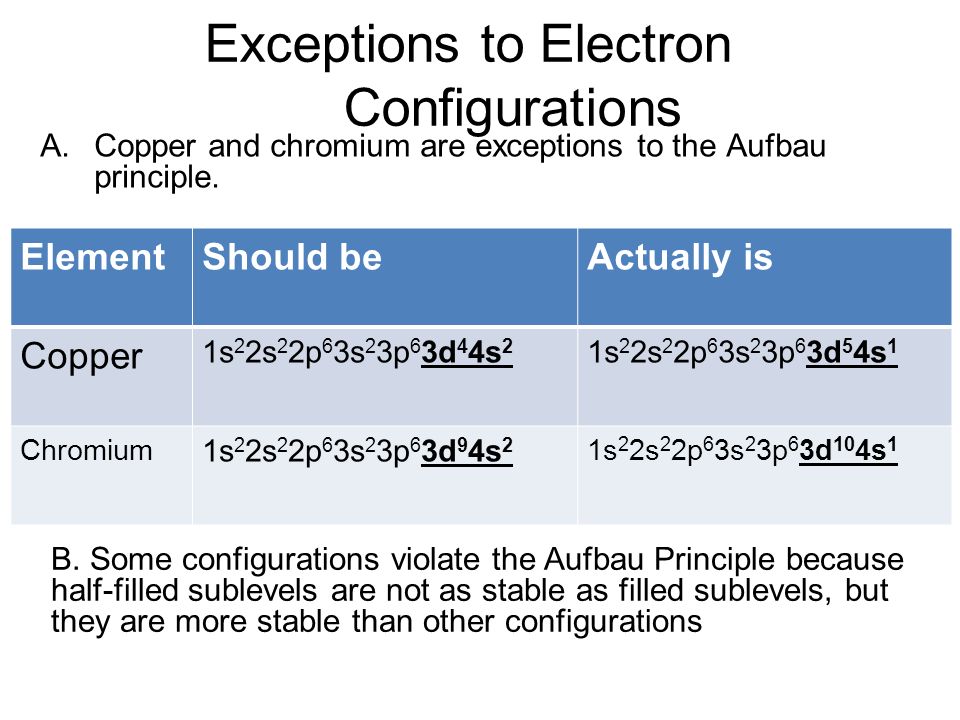 Aufbau Diagram Copper Wiring Diagram
Aufbau Diagram Copper Wiring Diagram
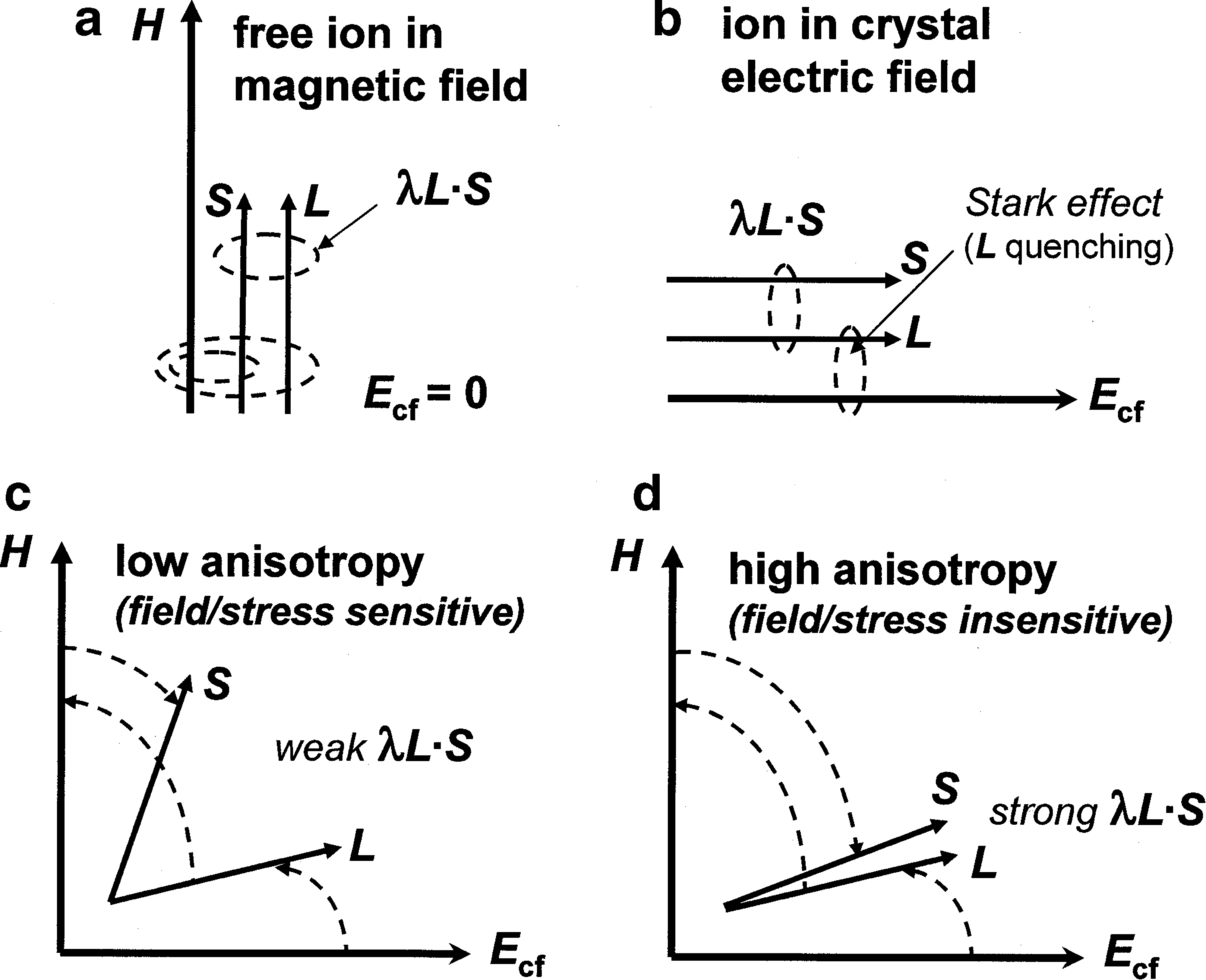 Magnetic Ions In Oxides Springerlink
Magnetic Ions In Oxides Springerlink
 Pauli Exclusion Principle An Overview Sciencedirect Topics
Pauli Exclusion Principle An Overview Sciencedirect Topics
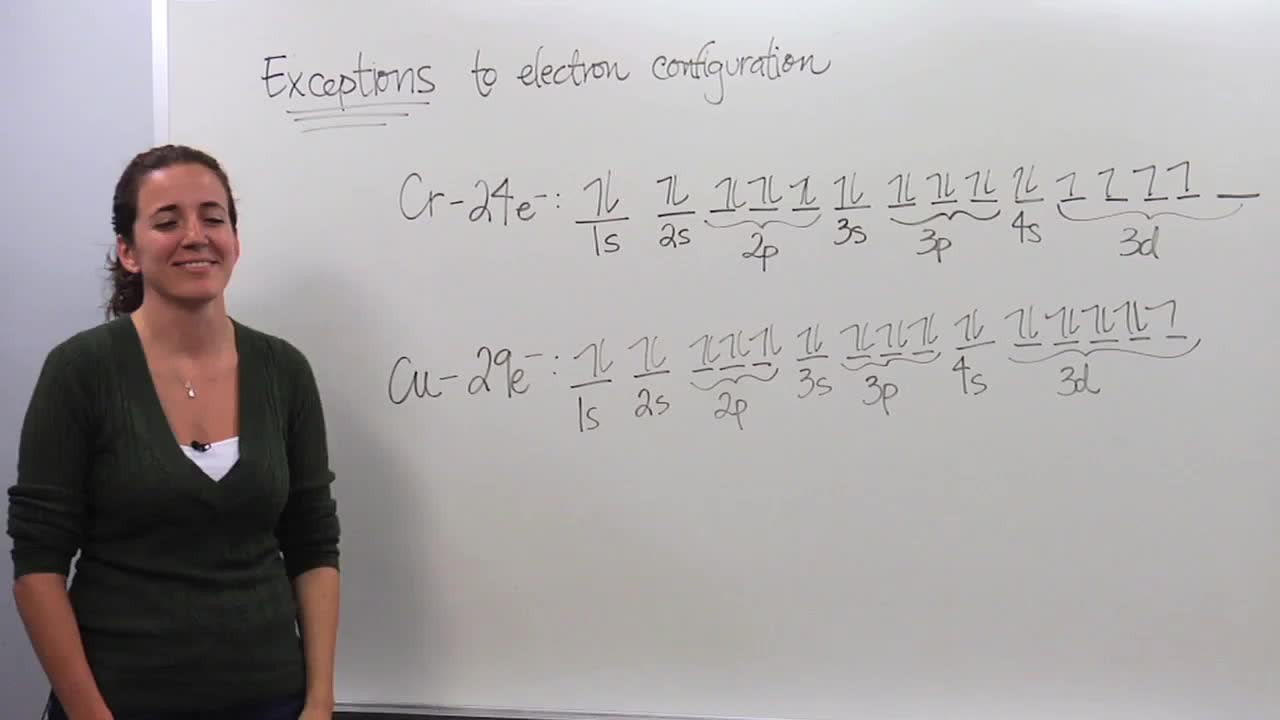 Exceptions To Electron Configuration Concept Chemistry Video By
Exceptions To Electron Configuration Concept Chemistry Video By
Acetylene Vinylidene And The Vinyl Cation In Ground And Excited
 Transition Metal Definition Properties Elements Facts
Transition Metal Definition Properties Elements Facts
 Exceptions To Electron Configuration Concept Chemistry Video By
Exceptions To Electron Configuration Concept Chemistry Video By
 Atomic Structures Pauli Exclusion Principle Aufbau Principle
Atomic Structures Pauli Exclusion Principle Aufbau Principle

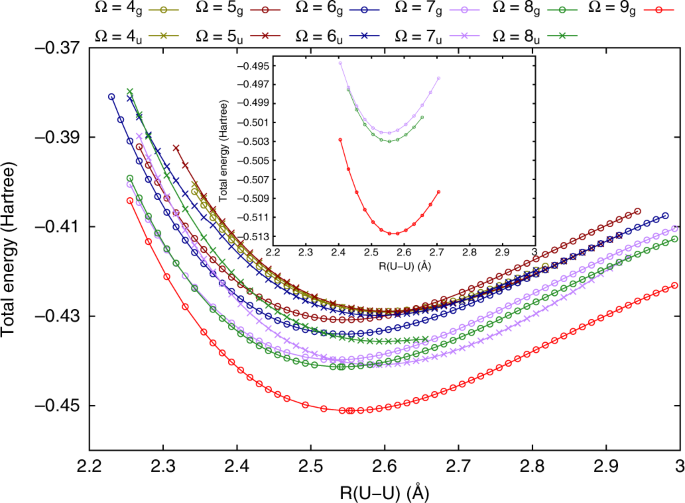 Relativistic Quantum Chemical Calculations Show That The Uranium
Relativistic Quantum Chemical Calculations Show That The Uranium
 Magnetic Ions In Oxides Springerlink
Magnetic Ions In Oxides Springerlink
0 Response to "What Is The Basis For Exceptions To The Aufbau Diagram"
Post a Comment