Examine The Following Phase Diagram And Determine What Phases Exists At Point A
A b temperature 760 torr f 18. H 2o h 2s.
7 First Order Phase Transitions
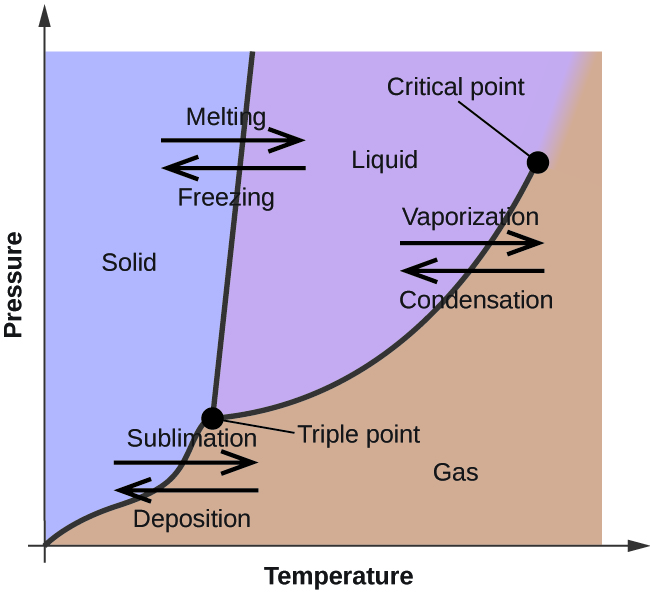
Phase diagrams depict the effects of temperature and pressure on phase changes.
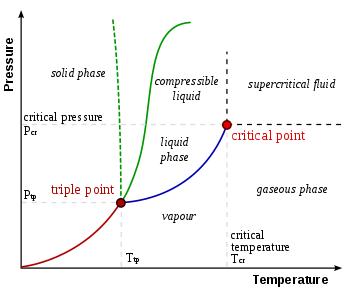
Examine the following phase diagram and determine what phases exists at point a. Examine the following phase diagram and determine what phase exists at point 760 som temperature a supercritical fluid b liquid c vaporliquid d vapor e solid the phase diagram of a substance i s given below. Critical point the point in temperature and pressure on a phase diagram where the liquid and gaseous phases of a substance merge together into a single phase. Neon condenses due to a dipole dipole forces.
Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first. C bo changes from a solid to a liquid as one follows the line from c to d. Examine the following phase diagram and determine what phase exists at point f.
A vapor liquid b vapor c liquid d solid e supercritical fluid 4. Examine the following phase diagram and determine what phase exists at point f. Beyond the temperature of the critical point the merged single phase is known as a supercritical fluid.
Examine the following phase diagram and identify the feature represented by point a and point b. Consider the following phase diagram and identify the process occurring as one goes from point c to point d. Phase diagrams are graphs of the relationship between the pressure and the temperature of a gas.
Ammonias unusually high melting point is the result of 20. Aincreasing temperature with a phase change from solid to liquid bincreasing temperature with a phase change from solid to vapor cincreasing temperature with a phase change from liquid to vapor dincreasing temperature with no phase change eincreasing temperature beyond the critical point7. A bos has a lower density than bol.
B increasing temperature with a phase change from solid to vapor examine the following phase diagram and determine what phase exists at point f. You can visualize the effects of temperature and pressure by using phase diagrams. Examine the following phase diagram and determine what phase exists at point f.
Consider the following phase diagram and identify the process occuring as one goes from point c to point d. B london dispersion forces. Answer to examine the following phase diagram and determine what phase exists at point fa vapor liquidb vaporc liquidd.
These diagrams show the solid liquid and gas phases. C 7h 16 c 5h 12 b. B the triple point for bo is at a higher temperature than the melting point for bo.
Ccl 4 cbr 4 c. Neon atoms are attracted to each other by a dipole dipole forces. Examine the phase diagram for the substance bogusium bo and select the correct statement.
 What Is The Project Life Cycle Mavenlink
What Is The Project Life Cycle Mavenlink
 10 4 Phase Diagrams Chemistry Libretexts
10 4 Phase Diagrams Chemistry Libretexts
 Phase Change Evaporation Condensation Freezing Melting
Phase Change Evaporation Condensation Freezing Melting
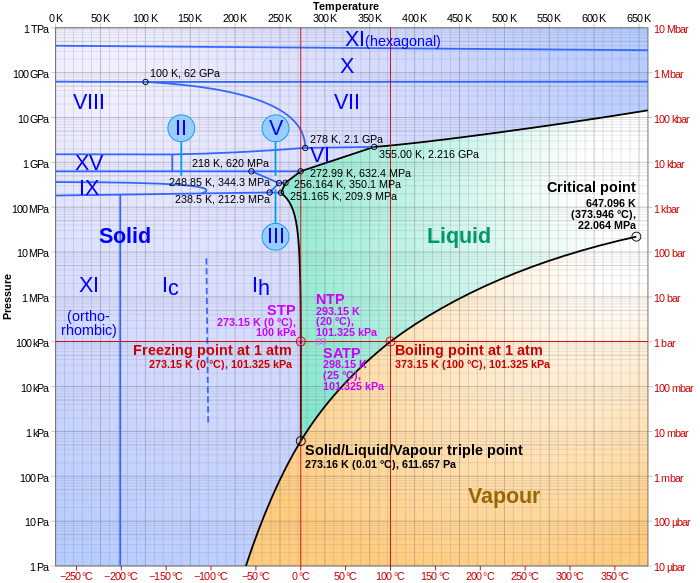
 Phase Diagrams Of Water Co2 Explained Chemistry Melting
Phase Diagrams Of Water Co2 Explained Chemistry Melting
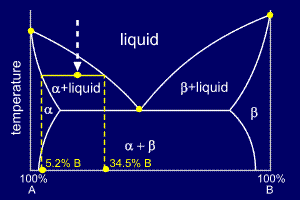
:max_bytes(150000):strip_icc()/eutectic-system-phase-diagram-56a135273df78cf7726863ea.png) Eutectic Definition And Examples
Eutectic Definition And Examples
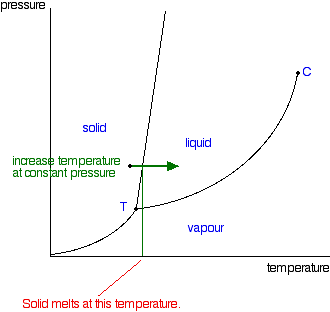 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
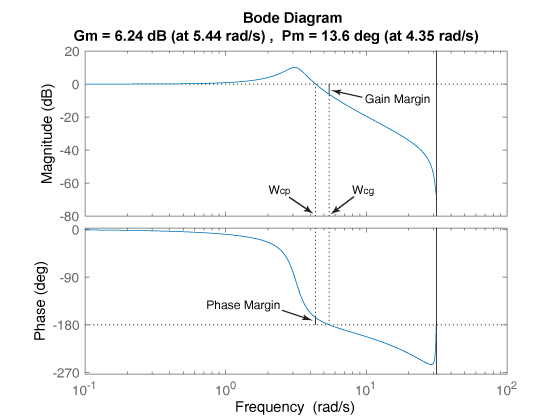
 Gain Margin Phase Margin And Crossover Frequencies Matlab Margin
Gain Margin Phase Margin And Crossover Frequencies Matlab Margin
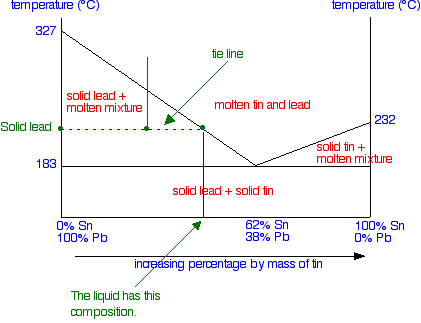 Solid Liquid Phase Diagrams Tin And Lead
Solid Liquid Phase Diagrams Tin And Lead
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
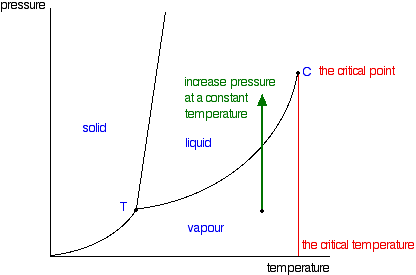 Phase Diagrams Of Pure Substances
Phase Diagrams Of Pure Substances
 Phase Diagrams An Overview Sciencedirect Topics
Phase Diagrams An Overview Sciencedirect Topics
Iron Iron Carbide Phase Diagram Example
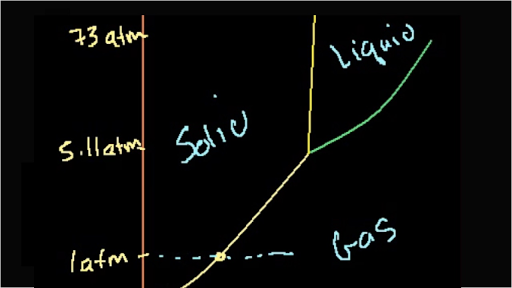
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
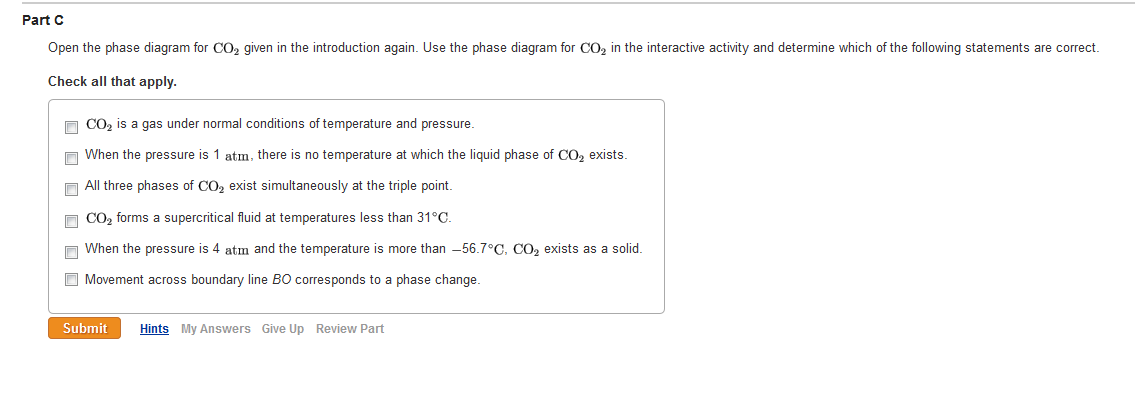 Solved Open The Phase Diagram For Co2 Given In The Introd
Solved Open The Phase Diagram For Co2 Given In The Introd


0 Response to "Examine The Following Phase Diagram And Determine What Phases Exists At Point A"
Post a Comment