Consider The Sugar Water Phase Diagram
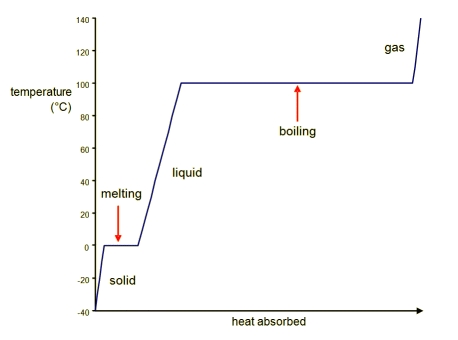
Sugar water syrup solution is one phase. Using figure 92 the pressuretemperature phase diagram for h 2 o determine the pressure to which the specimen must be raised or lowered to cause it a to melt and b to sublime.
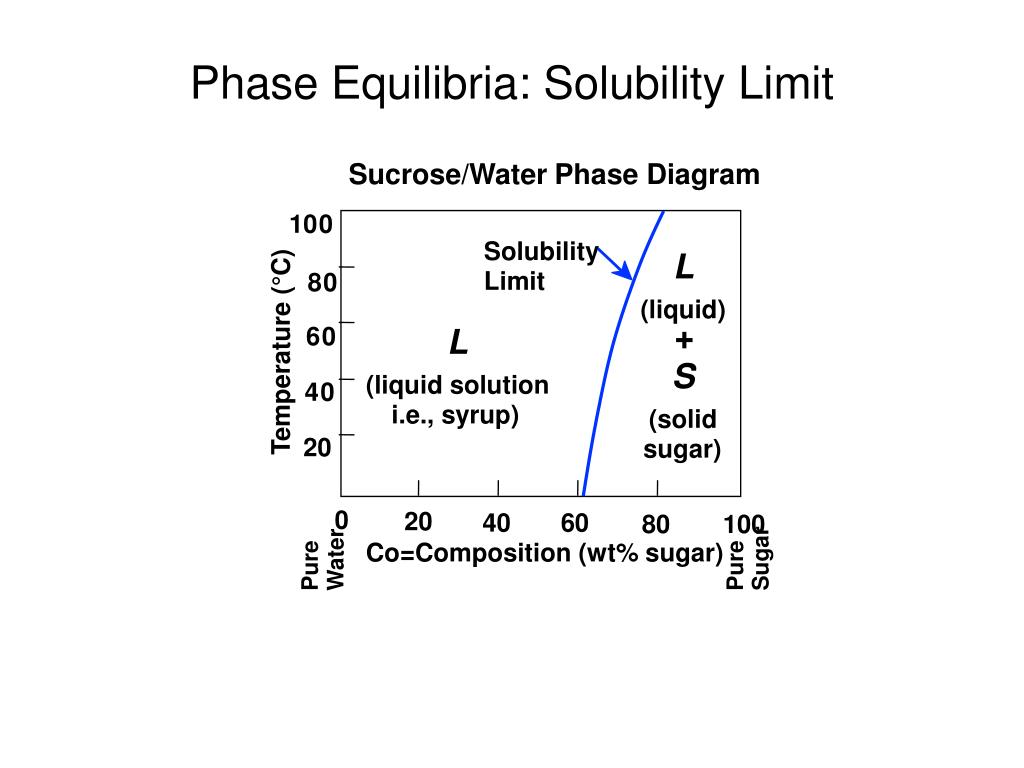
 Phase Equilibria Solubility Limit Sucrose Water Phase Diagram Pure
Phase Equilibria Solubility Limit Sucrose Water Phase Diagram Pure
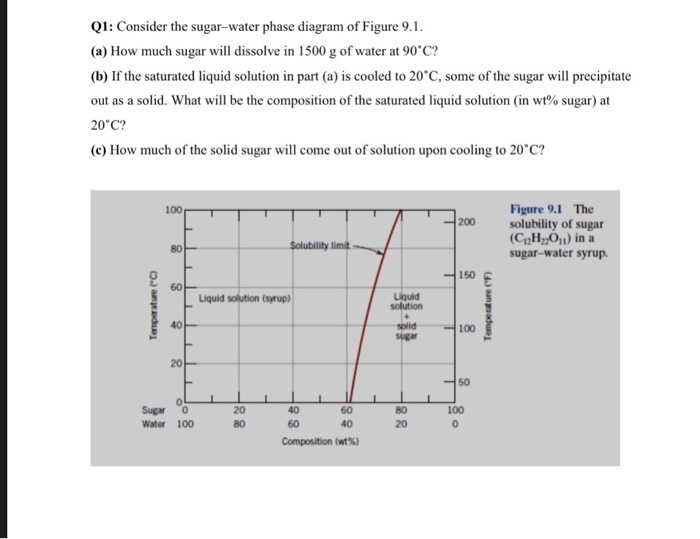
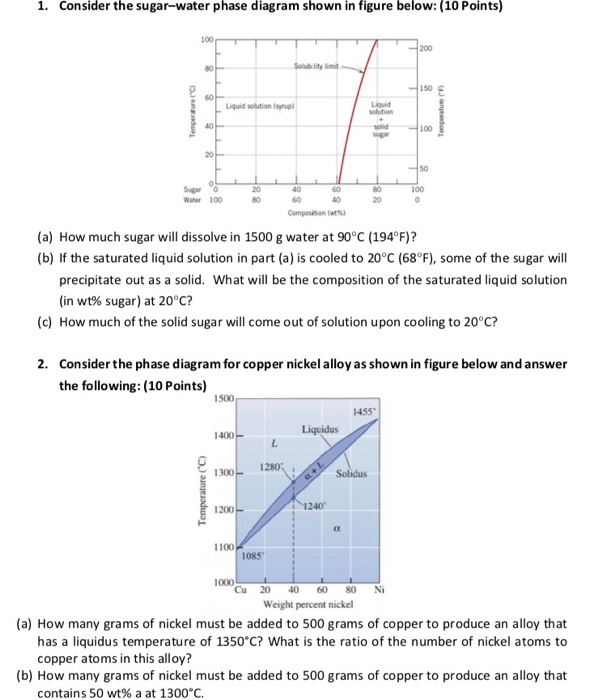
A how much sugar will dissolve in 1000 g of water at 90 c.
Consider the sugar water phase diagram. One component or unary phase diagrams 95 consider a specimen of ice that is at 15c and 10 atm pressure. One component or unary phase diagrams. A how much sugar will dissolve in 1500 g water at 90c 194f.
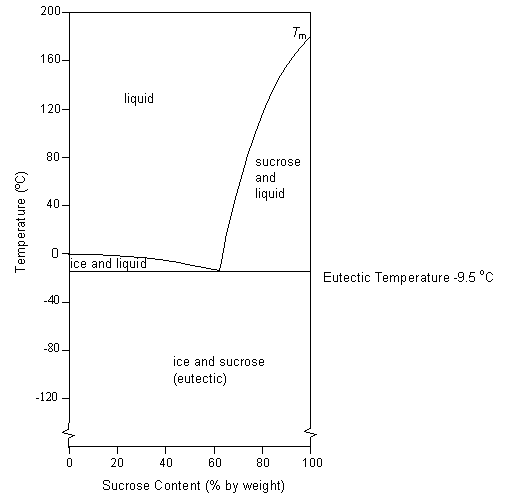
The solid sugar is another phase. A how much sugar will dissolve in 1500 g of water at 90c 194f. Consider the sugarwater phase diagram of figure 101.
B if the saturated liquid solution in part a is cooled to 20c 68f some of the sugar will precipitate out as a solid. B if the saturated liquid solution in part a is cooled to 20 c some of the sugar will precipitate out as a solid. B if the saturated liquid solution in part a is cooled to 20c 68f some of the sugar will precipitate out as a solid.
B if the saturated liquid solution in part a is cooled to 20c 68f some of the sugar will precipitate out as a solid. Consider the sugarwater phase diagram of figure. Phase diagrams problem solutions solubility limit 91 consider the sugarwater phase diagram of figure 91.
If two or more phases present in a given system a boundary separating the phases will exist across. Using figure 92 the pressuretemperature phase diagram for determine the pressure to which the specimen must be raised or lowered to cause it a to melt and b to sublime. A how much sugar will dissolve in 1500 g water at 90 c 194 f.
95 consider a specimen of ice that is at and 10 atm pressure. Consider the sugarwater phase diagram of figure 101. A how much sugar will dissolve in 1500 g water at 90 c 194 f.
Figure 101 the solubility of sugar c 12 h 22 o 11 in a sugarwater syrup. Consider the sugar water phase diagram. A how much sugar will dissolve in 1500 g water at 90c 194f.
Every solid liquid and gaseous solution may be considered a phase. B if the saturated liquid solution in part a is cooled to 20c 68f some of the sugar will precipitate out as a solid. B if the saturated liquid solution in part a is cooled to 20 c 68 f some of the sugar will precipitate out as a solid.
Every pure material is considered to be a phase. 14440407 ch9 question 91 consider the sugarwater phase diagram of figure 91. A how much sugar will dissolve in 1000 g of water at 80c 176f.
B if the saturated liquid solution in part a is cooled to 20 c 68 f some of the sugar will. 91 consider the sugarwater phase diagram of figure 91.
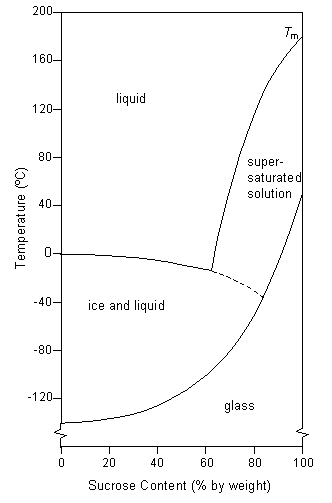 Doitpoms Tlp Library Avoidance Of Crystallization In Biological
Doitpoms Tlp Library Avoidance Of Crystallization In Biological
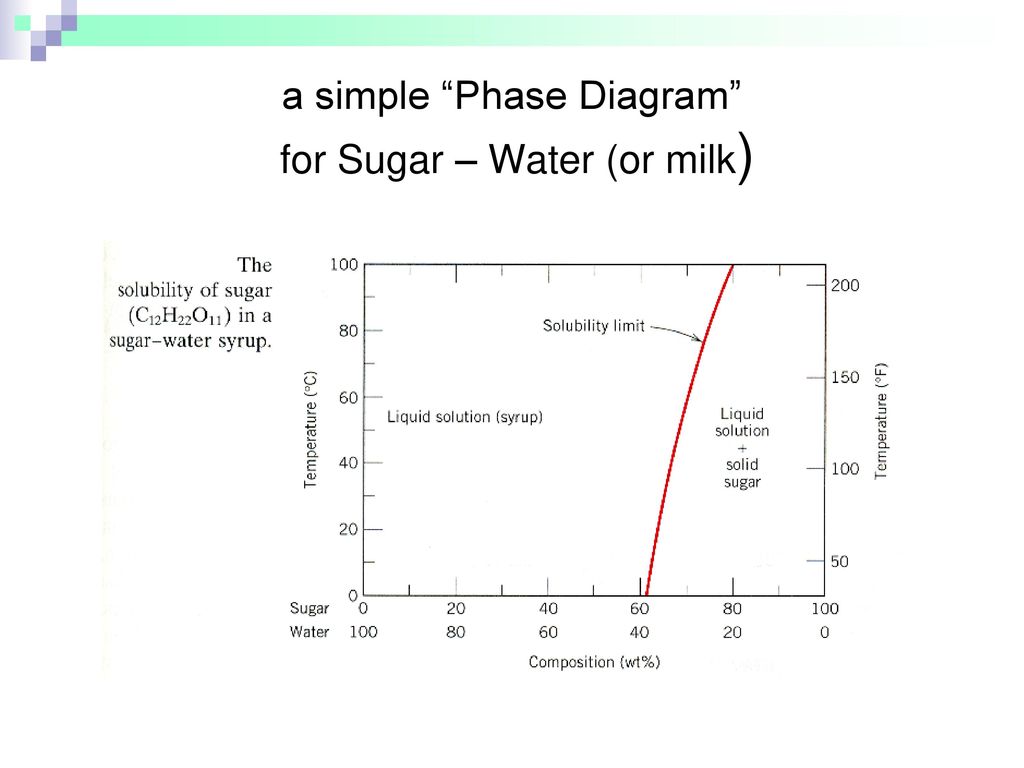 Part 6 Chemistry Engineering Department 23 10 Ppt Download
Part 6 Chemistry Engineering Department 23 10 Ppt Download
 Doitpoms Tlp Library Avoidance Of Crystallization In Biological
Doitpoms Tlp Library Avoidance Of Crystallization In Biological
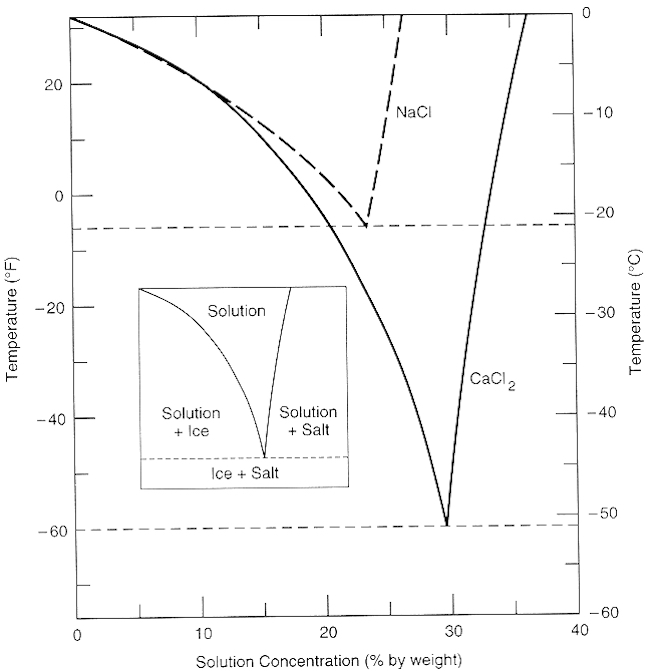 Solutions Solubility And Colligative Properties Chemistry
Solutions Solubility And Colligative Properties Chemistry
 The Sugar Water Phase Diagram For The Sugar Water System With
The Sugar Water Phase Diagram For The Sugar Water System With
 Chapter 9 Sections 9 2 9 3 9 4 Ppt Video Online Download
Chapter 9 Sections 9 2 9 3 9 4 Ppt Video Online Download
 Spacechem 2013 Tournament Part 17 Other Things Phases Of
Spacechem 2013 Tournament Part 17 Other Things Phases Of
 Chapter 9 Phase Diagrams Materials Science And Engineering 201
Chapter 9 Phase Diagrams Materials Science And Engineering 201
Teach Yourself Phase Diagrams And Phase Transformations
 Phase Changes Boundless Chemistry
Phase Changes Boundless Chemistry
 Solved Consider The Sugar Water Phase Diagram Of Figure 9 1 A
Solved Consider The Sugar Water Phase Diagram Of Figure 9 1 A
 L3 Phase Diagrams And Solidification 2013 Pdf L3 Phase Diagrams
L3 Phase Diagrams And Solidification 2013 Pdf L3 Phase Diagrams
 Ps7 Solutions E 344 Spring 2015 Problem Set 7 Binary Phase
Ps7 Solutions E 344 Spring 2015 Problem Set 7 Binary Phase
 Water Phase Diagram Phase Diagram For Water Youtube
Water Phase Diagram Phase Diagram For Water Youtube
Phase Diagrams Mse280 Pages 1 24 Text Version Fliphtml5
 Ppt Phase Equilibria Solubility Limit Powerpoint Presentation
Ppt Phase Equilibria Solubility Limit Powerpoint Presentation
 Solved Consider The Sugar Water Phase Diagram Of Fig 9 1
Solved Consider The Sugar Water Phase Diagram Of Fig 9 1
 Water Activity Of Multiple Emulsions With Respect To The Sugar
Water Activity Of Multiple Emulsions With Respect To The Sugar
 The Sugar Water Phase Diagram For The Sugar Water System With
The Sugar Water Phase Diagram For The Sugar Water System With
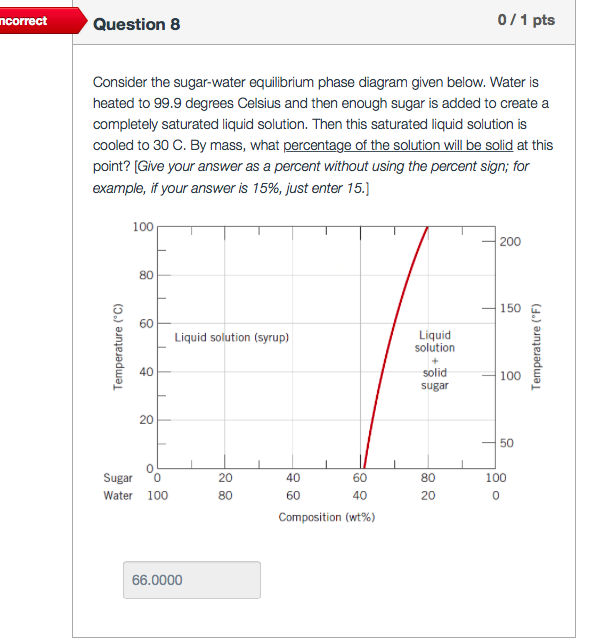 Solved Consider The Sugar Water Equilibrium Phase Diagram
Solved Consider The Sugar Water Equilibrium Phase Diagram

0 Response to "Consider The Sugar Water Phase Diagram"
Post a Comment