What Is The Activation Energy For The Reaction In This Energy Diagram
The activation energy for the reverse reaction is 500100 kj 400 kj. The activation energy is what determines the kinetics of a reaction.
Reaction Energy Profiles Activation Energy Exothermic Endothermic
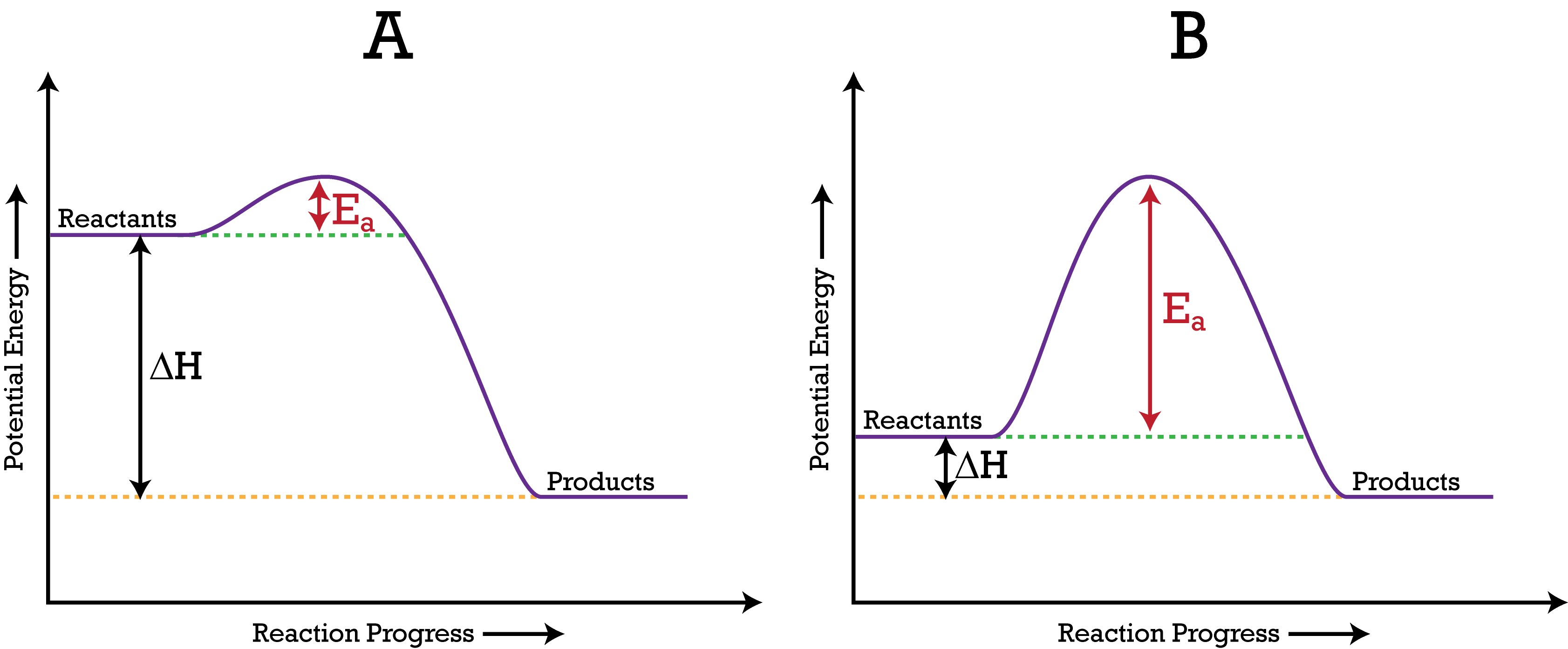
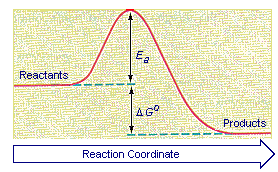
In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled.

What is the activation energy for the reaction in this energy diagram. But before the reactants can be converted into products the free energy of the system must overcome the activation energy for the reaction as shown in the figure below. At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products. The higher the energy hill the slower the reaction.
In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. The enthalpy change for the forward reaction is 100200 kj 100 kj. Select all that apply a catalyst lowers the activation energy.
The energy diagram would look something like this. The activation energy shown in the diagram below is for the forward reaction reactants products which is exergonic. The activation energy for the forward reaction is 500200 kj 300 kj.
The vertical axis in this diagram represents the free energy of a pair of molecules as a chlorine atom is transferred from one to the other. This assumes that the reaction proceeds back and forth via the same steps. This state is also known as an activated complex.
At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products. Activation energy is the difference between the starting amount of energy of the reactants and the energy of the activated complex. If the reaction were to proceed in the reverse direction endergonic the transition state would remain the same but the activation energy would be larger.
At the very top of the energy barrier the reaction is at its transition state ts which is the point at which the bonds are in the process of breaking and forming. The energy of the reactants of the reverse reaction is 13 kj the activated complex is still at 83 kj. This state is also known as an activated complex.
 What Is The Activation Energy For A Reverse Reaction Quora
What Is The Activation Energy For A Reverse Reaction Quora
 What Is The Enthalpy Of Reaction From The Following Energy Diagram
What Is The Enthalpy Of Reaction From The Following Energy Diagram
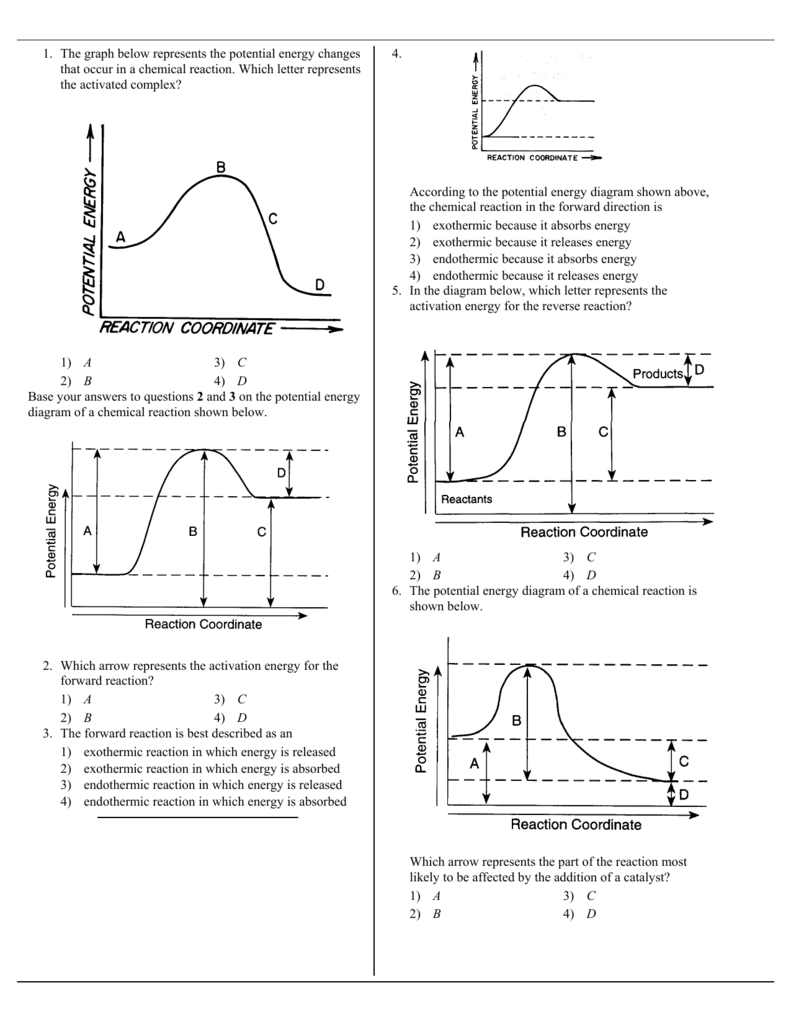 1 The Graph Below Represents The Potential Energy
1 The Graph Below Represents The Potential Energy
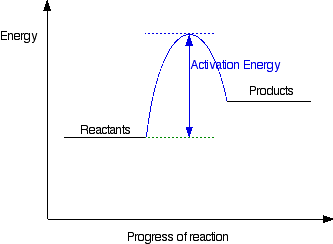 A Look At Energy Profiles For Reactions Chemistry Libretexts
A Look At Energy Profiles For Reactions Chemistry Libretexts
Energy Diagram Module Series Part Three Intermediates And Rate
 How To Read Potential Energy Diagrams
How To Read Potential Energy Diagrams
 Energy Diagram For A Simple Reaction The Same Reaction With Lowered
Energy Diagram For A Simple Reaction The Same Reaction With Lowered
 Activation Energy In Chemical Reactions
Activation Energy In Chemical Reactions
 Reaction Energy Diagram Sn1 Youtube
Reaction Energy Diagram Sn1 Youtube
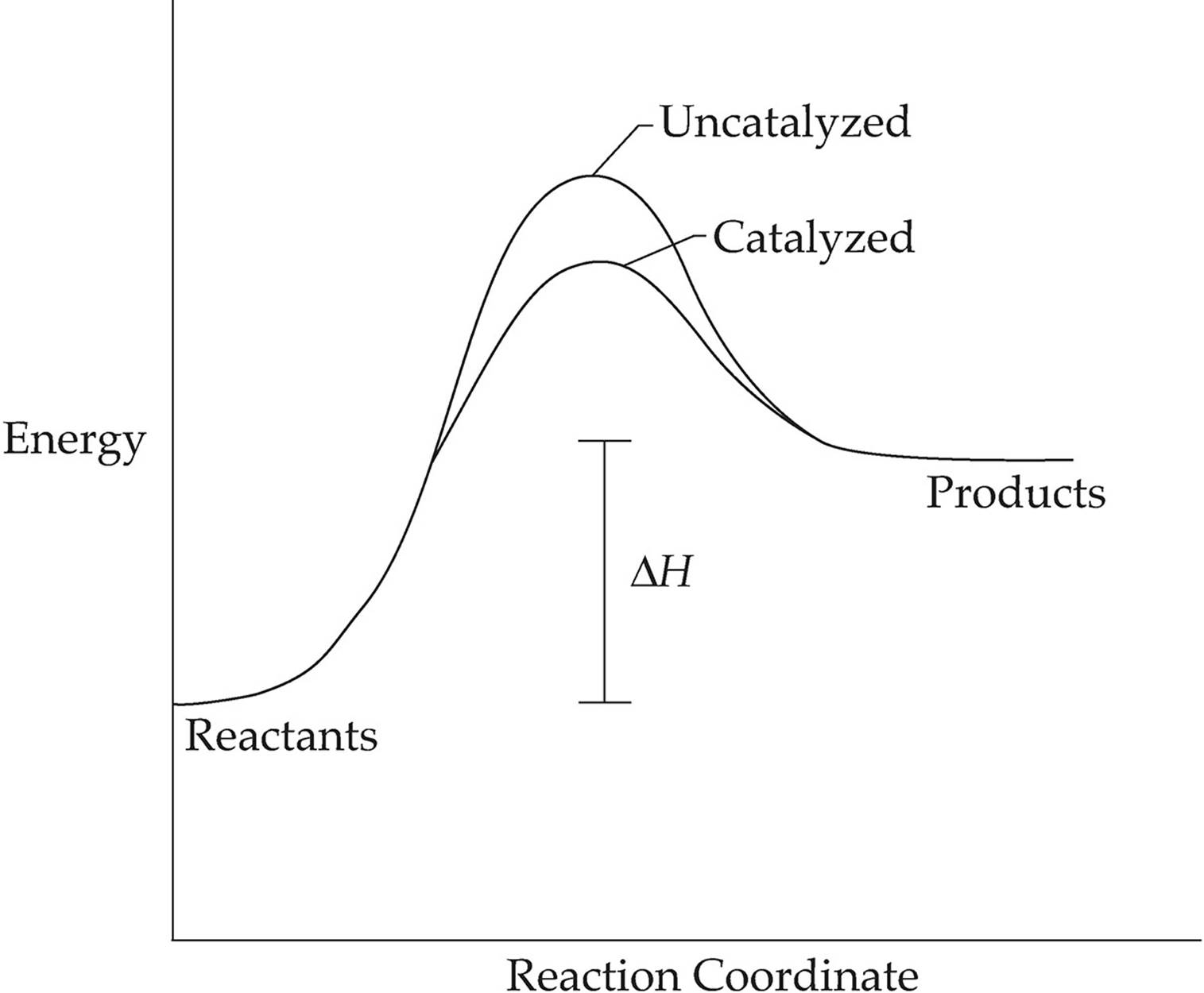 Catalysts And Energy Diagrams Chemical Reactions Energy Changes
Catalysts And Energy Diagrams Chemical Reactions Energy Changes
 Transition State Theory Chemistry Britannica Com
Transition State Theory Chemistry Britannica Com
 Activation Energy Article Enzymes Khan Academy
Activation Energy Article Enzymes Khan Academy

Organometallic Hypertextbook The Principle Of Microscopic Reversibility
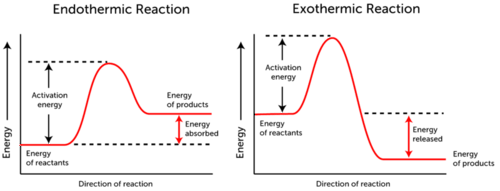
 What Is Activation Energy For The Forward Reaction Represented In
What Is Activation Energy For The Forward Reaction Represented In
 Activation Energy Higher Chemistry Unit 1
Activation Energy Higher Chemistry Unit 1
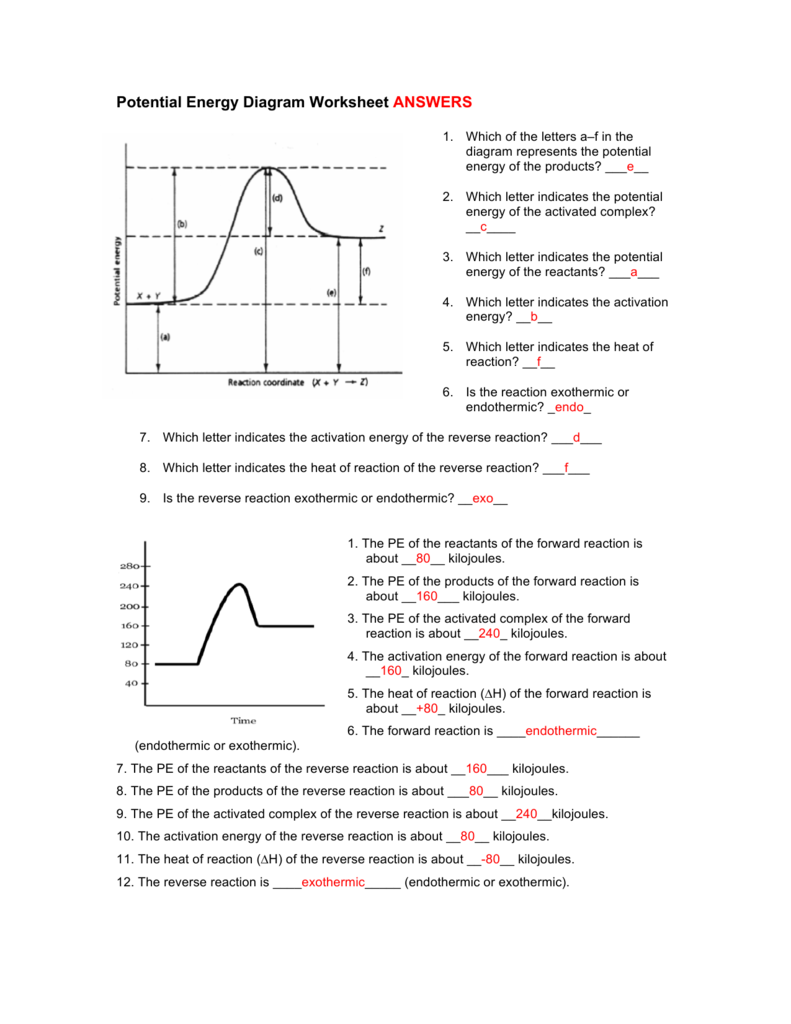 Potential Energy Diagram Worksheet Answers
Potential Energy Diagram Worksheet Answers
 A Reaction Is Endothermic With H 100 Kj Mol If The Activation
A Reaction Is Endothermic With H 100 Kj Mol If The Activation
Mcat General Chemistry Question 62 Answer And Explanation Maintests Com
What Is The Activation Energy For A Reverse Reaction Quora
 Mechanism Of Reaction And Catalysis Rate And Extent Of Reaction
Mechanism Of Reaction And Catalysis Rate And Extent Of Reaction
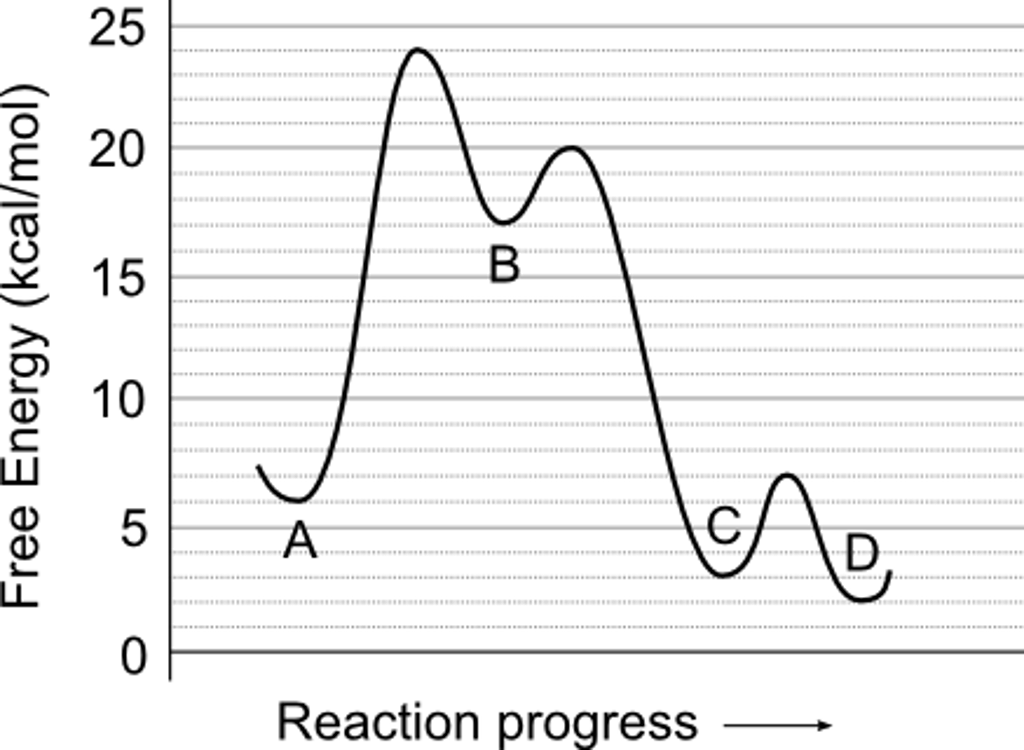 Solved 1 Use The Reaction Energy Diagram Above To Answer
Solved 1 Use The Reaction Energy Diagram Above To Answer
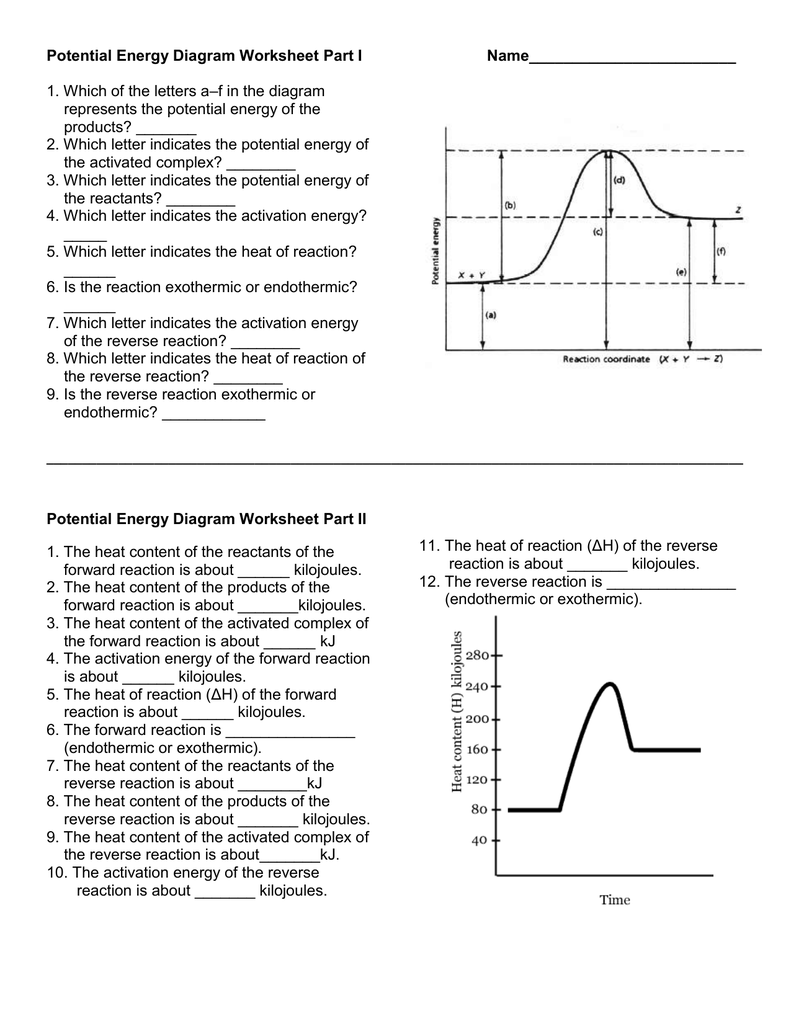 Potential Energy Diagram Worksheet Part I
Potential Energy Diagram Worksheet Part I



0 Response to "What Is The Activation Energy For The Reaction In This Energy Diagram"
Post a Comment