For This Question Consider The Figure To The Right Shows An Energy Level Diagram
One star is red while the other star is blue. Diagram with 5 energy levels labeled 0 ev 05 ev 10 ev 50 ev and 10 ev from top to bottom a hypothetical hydrogen like atom has energy states as represented in the energy level diagram above.
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
According to bohrs theory electrons of an atom revolve around the nucleus on certain orbits or electron shells.

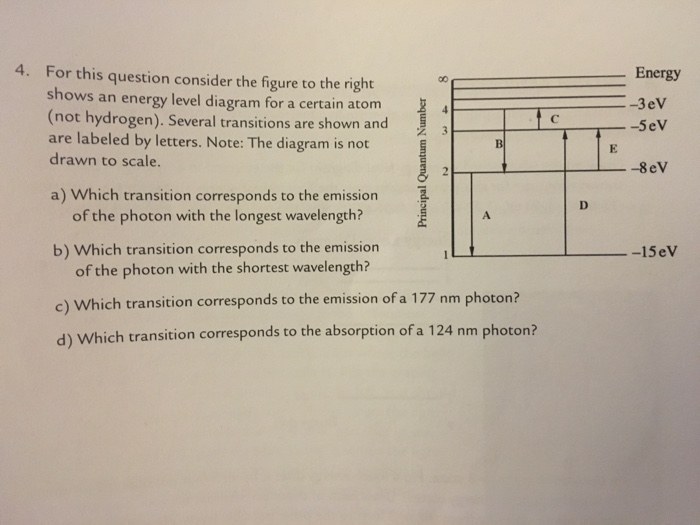
For this question consider the figure to the right shows an energy level diagram. Complementary angles are angles which are equal to 90 degrees wherein when you add both angles which are obviously 45 degrees the sum is 90 degrees. It is because they both had the same vertex which is point g. Angle cgb is complementary to angle agd.
Macro exam 111213 the figure in the window on the right shows average annual growth rates in real gdp per hour worked in the united states. A which transition corresponds to the absorption of the photon with the longest wavelength. To wit the particle will move toward point of lower potential energy to the right a particle is in equilbrium when its potential energy is minimum and it will move in that direction if released from a position of higher pot.
Several transitions are shown and are labeled by letters. One night you look up in the sky and notice that two of the brightest visible stars have distinctly different colors. At energy level 2 there are both s and p orbitals with the 2s having lower energy than the 2p.
What can be proven true in the diagram is the statement in letter c. This is an exception to what you may have thought but its whats observed in nature. For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen.
Wholsit consider poetry for a career cause it sure aint going to be physics. This is because the electrons on the orbit are captured. Several transitions are shown and are labeled by letters.
The atom is in the ground state when it absorbs a photon with frequency 218 x 1015 hz. For this question consider the figure to the right shows an energy level diagram for a certain atom not hydrogen. Each orbit has its specific energy level which is expressed as a negative value.
The diagram is not drawn to scale. What is the energy of the photon emitted when the electron in a hydrogen atom drops from energy level e5 to energy level e2. Notice that the 4s has lower energy than the 3d.
In this section we will discuss the energy level of the electron of a hydrogen atom and how it changes as the electron undergoes transition. Energy levels 3 4 and 5 are also shown. The figure shown above shows the energy levels for a hydrogen atom.
The three 2p subshells are represented by three dashes of the same energy. The electron can drop from level n 3 to level n 2 and in so doing emit hα which is a balmer series visible photon.
 Figure 8 From Luminescence Nanothermometry Semantic Scholar
Figure 8 From Luminescence Nanothermometry Semantic Scholar
 Formation Of Spectral Lines Astronomy
Formation Of Spectral Lines Astronomy
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy
Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy
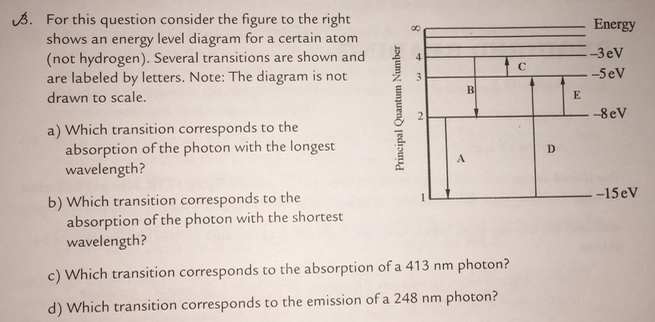 Solved For This Question Consider The Figure To The Right
Solved For This Question Consider The Figure To The Right
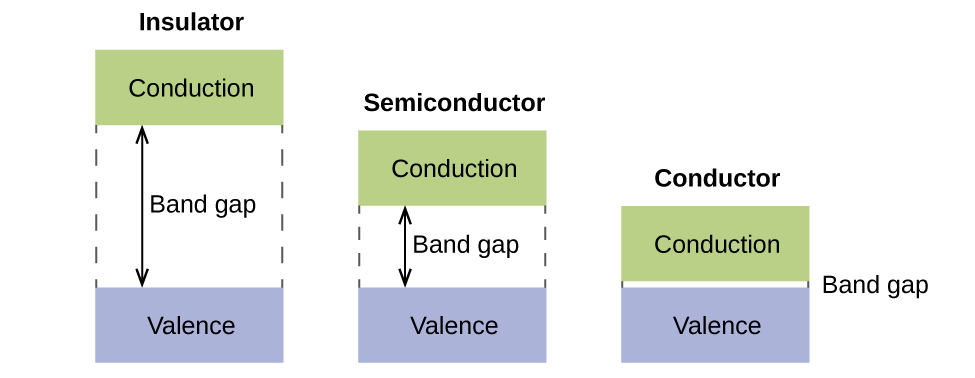
 Energy Band Diagram An Overview Sciencedirect Topics
Energy Band Diagram An Overview Sciencedirect Topics
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Figure 1 From Iii Bloch Nuclides Iwamura Transmutations
Figure 1 From Iii Bloch Nuclides Iwamura Transmutations
 Formation Of Spectral Lines Astronomy
Formation Of Spectral Lines Astronomy
Osa Probing Quantifying And Freezing Coherence In A Thermal
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Schematic Drawing Of The Energy Level Diagram Of An Organic Material
Schematic Drawing Of The Energy Level Diagram Of An Organic Material
 How To Represent Electrons In An Energy Level Diagram Dummies
How To Represent Electrons In An Energy Level Diagram Dummies
 Germany S Energy Consumption And Power Mix In Charts Clean Energy Wire
Germany S Energy Consumption And Power Mix In Charts Clean Energy Wire
 The Economy Unit 2 Technology Population And Growth
The Economy Unit 2 Technology Population And Growth
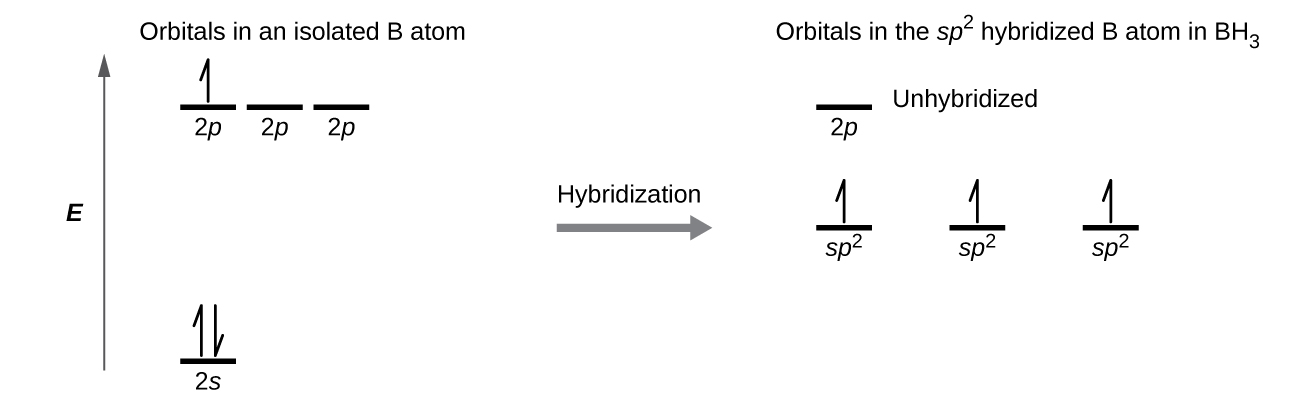 8 2 Hybrid Atomic Orbitals Chemistry
8 2 Hybrid Atomic Orbitals Chemistry
 9 8 Second Row Diatomic Molecules Chemistry Libretexts
9 8 Second Row Diatomic Molecules Chemistry Libretexts
 Energy Level And Transition Of Electrons Brilliant Math Science Wiki
Energy Level And Transition Of Electrons Brilliant Math Science Wiki
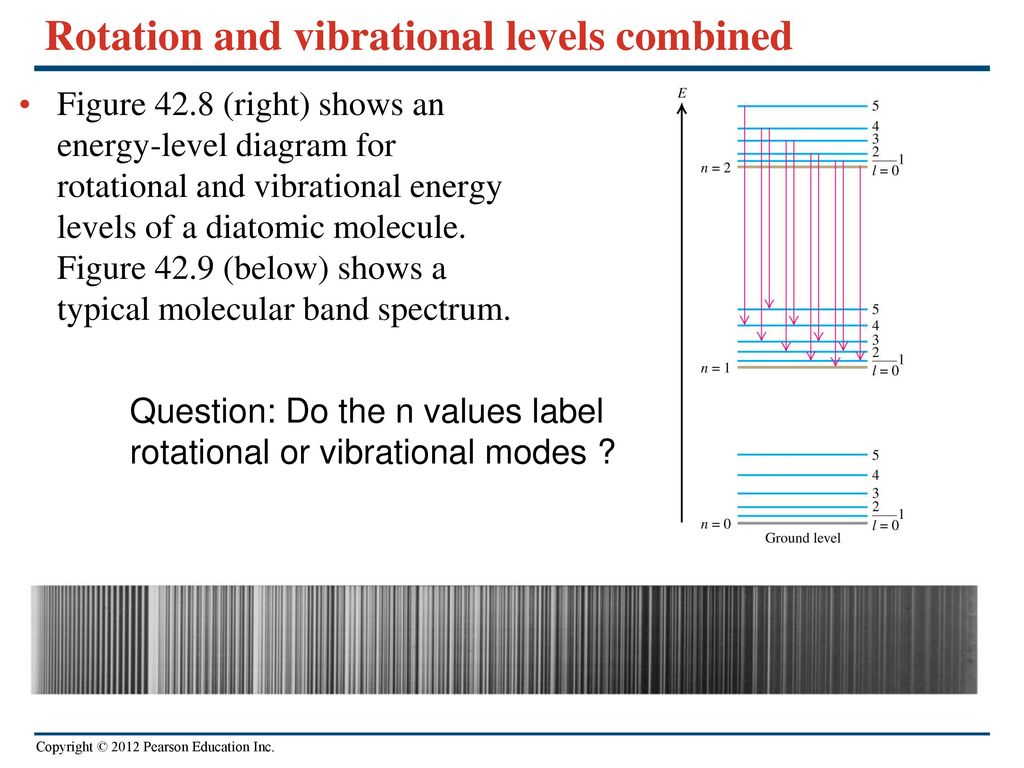 Question On Van Der Waals Interactions Ppt Download
Question On Van Der Waals Interactions Ppt Download
 What Are Fluorescence And Phosphorescence Education
What Are Fluorescence And Phosphorescence Education
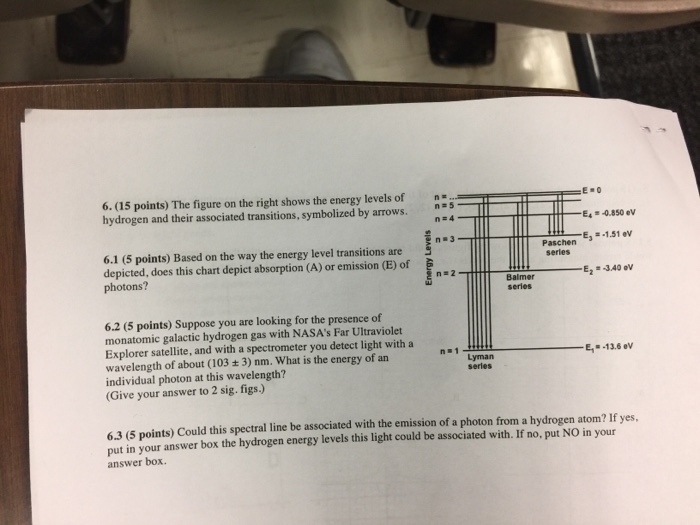

0 Response to "For This Question Consider The Figure To The Right Shows An Energy Level Diagram"
Post a Comment