One Way To Increase The Volume Of The Gas In The Balloon In The Diagram Above Is To
That the particles of an ideal gas. If the temperature of a gas remains constant but pressure is decreased the volume will a.
How could he now study the effects of.
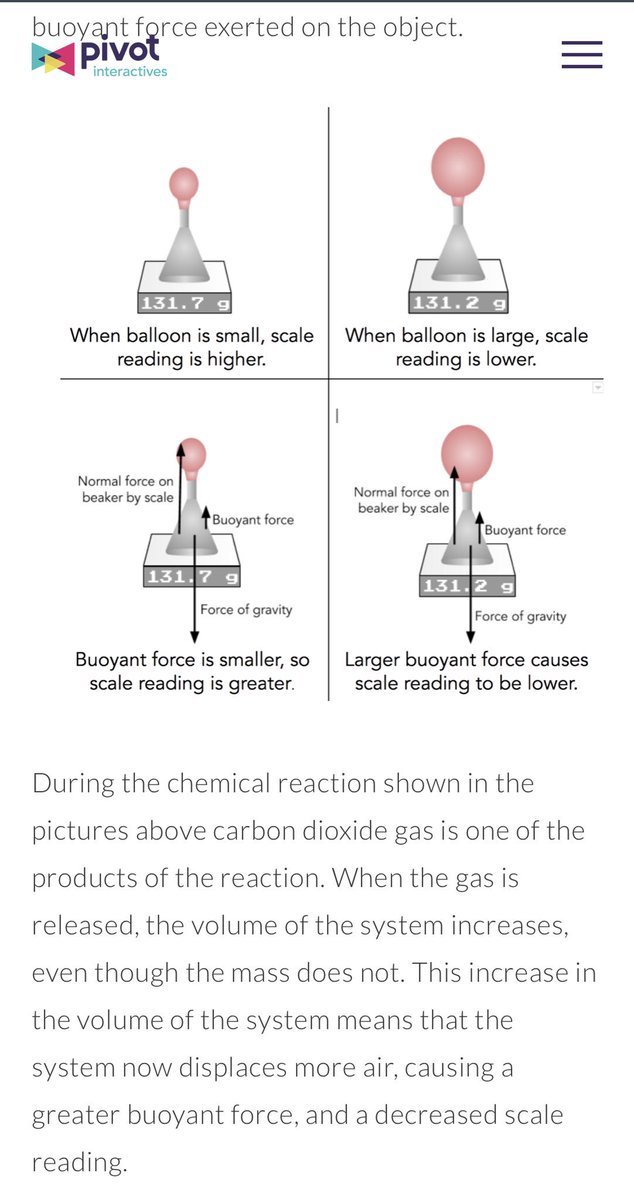
One way to increase the volume of the gas in the balloon in the diagram above is to. We increase the density of a balloon when we squeeze it and likewise increase air dentiy in the cylinder of a tire pump when we push the piston downward. One way to increase the volume of the gas in the balloon in the diagram below is to a. One way to increase the volume of the gas in the balloon in the diagram to the left is to a.
Increase the temperature of the water c. Cool the gas and the balloon only b. Push the balloon farther down into the water bath d.
Triple the density triple the pressure. Kinetic molecular theory of gases is. Push the balloon farther down into the water bath d.
Above is to f. Remain the same 7. Seal the top of the water bath.
Increase the temperature of the water h. Gas expands decrease in internal energy since no heat transfer in thus loss of ke pressure and temperature increase 2 temperature will increase because temperature and volume proportional. Seal the top of the water bath 6.
If the balloon is under a constant internal pressure calculate its volume at a temperature of 12ºc. Seal the top of the water bath 6. And to analyze a.
If a gases volume is decreased and pressure is constant its temperature will a. The volume of a balloon is 30 litres at a temperature of 27ºc. Increase the temperature of the water c.
Remain the same 8. Cool the gas in the balloon only. So pressure is proportional to density.
Chapter 25 ideal gas laws exercise 139 page 303 1. One way to increase the volume of the. That result can be achieved in three ways.
Gas in the balloon in the diagram. If the volume of gas in the balloon remains constant then an increase in temperature would result in an increased gas pressure in a balloon. 1 increase in volume thus decrease in pressure gas doing work on something thus loses internal energy according to 1st law thermo adiabatic insulated.
Convert 23 atm into mmhg. One of the main assumptions of the. Push the balloon farther down into the.
Experiment to determine how one variable affects the other. Calculate the gas temperature increase the vessel can withstand. Cool the gas and the balloon only b.
3 trial volume pressure temperature 1 100 ml 250 mm hg 298 k 2 300 ml 83 mm hg 298 k 3 500 ml 50 mm hg 298 k a student wants to study the effects of volume on gas pressureduring his experiment he recorded the above data. The density of air can also be doubled by simply compressing the air to half its volume. One of the main assumptions of the kinetic molecular theory.
 Forces And Pressure S Cool The Revision Website
Forces And Pressure S Cool The Revision Website
 How Do Hot Air Balloons Work Explain That Stuff
How Do Hot Air Balloons Work Explain That Stuff
:max_bytes(150000):strip_icc()/GettyImages-91560144-56a133d53df78cf772685abd.jpg) 3 Ways To Increase The Pressure Of A Gas
3 Ways To Increase The Pressure Of A Gas

 Charles S Law Video Ideal Gas Equation Khan Academy
Charles S Law Video Ideal Gas Equation Khan Academy
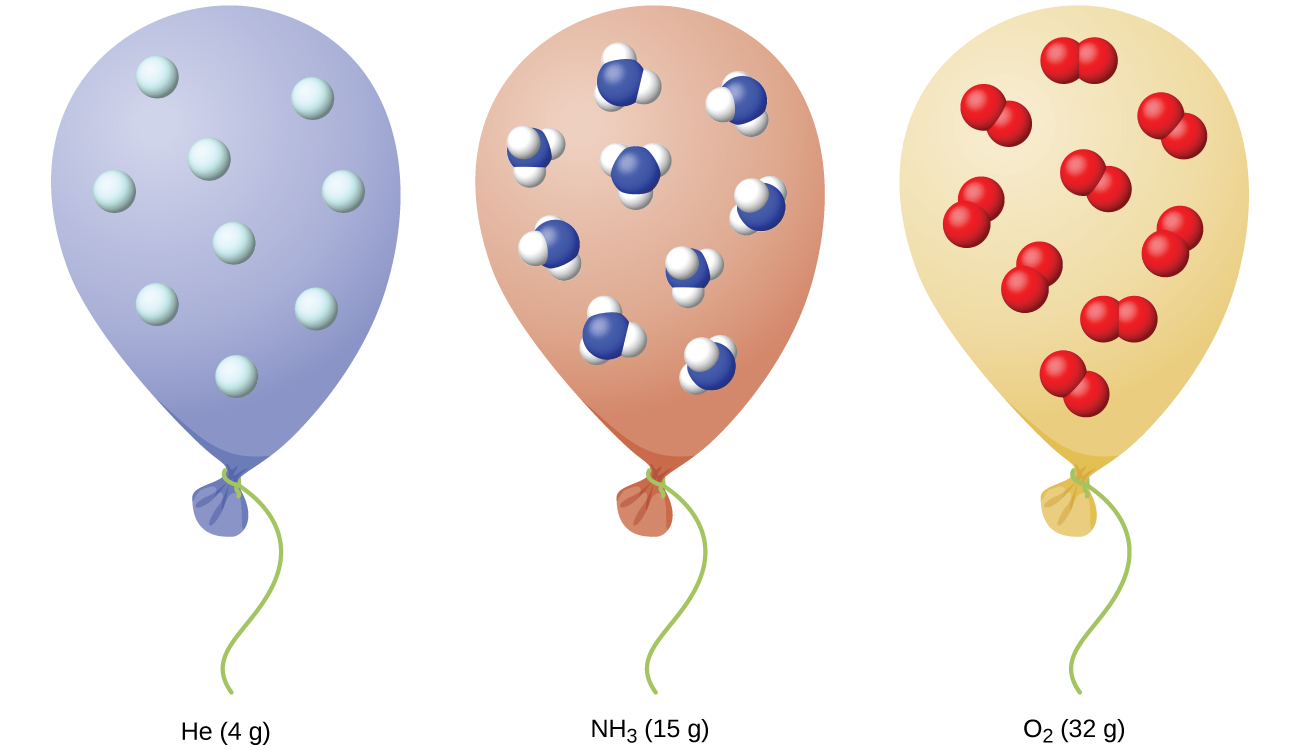 9 2 Relating Pressure Volume Amount And Temperature The Ideal
9 2 Relating Pressure Volume Amount And Temperature The Ideal
 New Design Simulation For A High Altitude Dual Balloon System To
New Design Simulation For A High Altitude Dual Balloon System To
 Ideal Gas Laws Ideal Gases Siyavula
Ideal Gas Laws Ideal Gases Siyavula
 Ideal Gas Laws Ideal Gases Siyavula
Ideal Gas Laws Ideal Gases Siyavula
 Compressing Gases Gases Hold Huge Amounts Of Energy And Their
Compressing Gases Gases Hold Huge Amounts Of Energy And Their
 Relating Pressure Volume Amount And Temperature The Ideal Gas
Relating Pressure Volume Amount And Temperature The Ideal Gas
 Chapter 2b Pure Substances Ideal Gas Updated 1 17 11
Chapter 2b Pure Substances Ideal Gas Updated 1 17 11
 Multi And Instabilities In Gas Partitioning Between Nanoporous
Multi And Instabilities In Gas Partitioning Between Nanoporous
The First Law Of Thermodynamics
 Mr Drew Hutcheson On Twitter I Love A Chem Demo With Nontoxic
Mr Drew Hutcheson On Twitter I Love A Chem Demo With Nontoxic
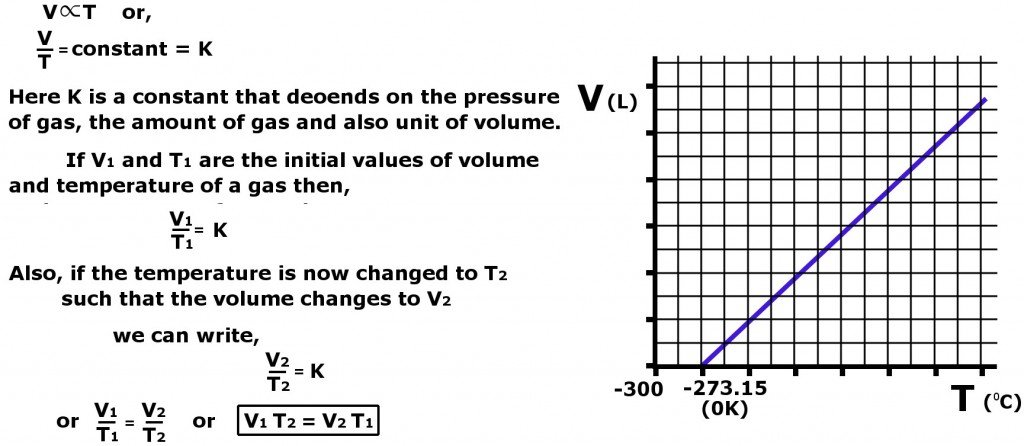 Charles Law Definition Explanation Formula And Equation
Charles Law Definition Explanation Formula And Equation
 Artificial Clouds And Inflammable Air The Science And Spectacle Of
Artificial Clouds And Inflammable Air The Science And Spectacle Of
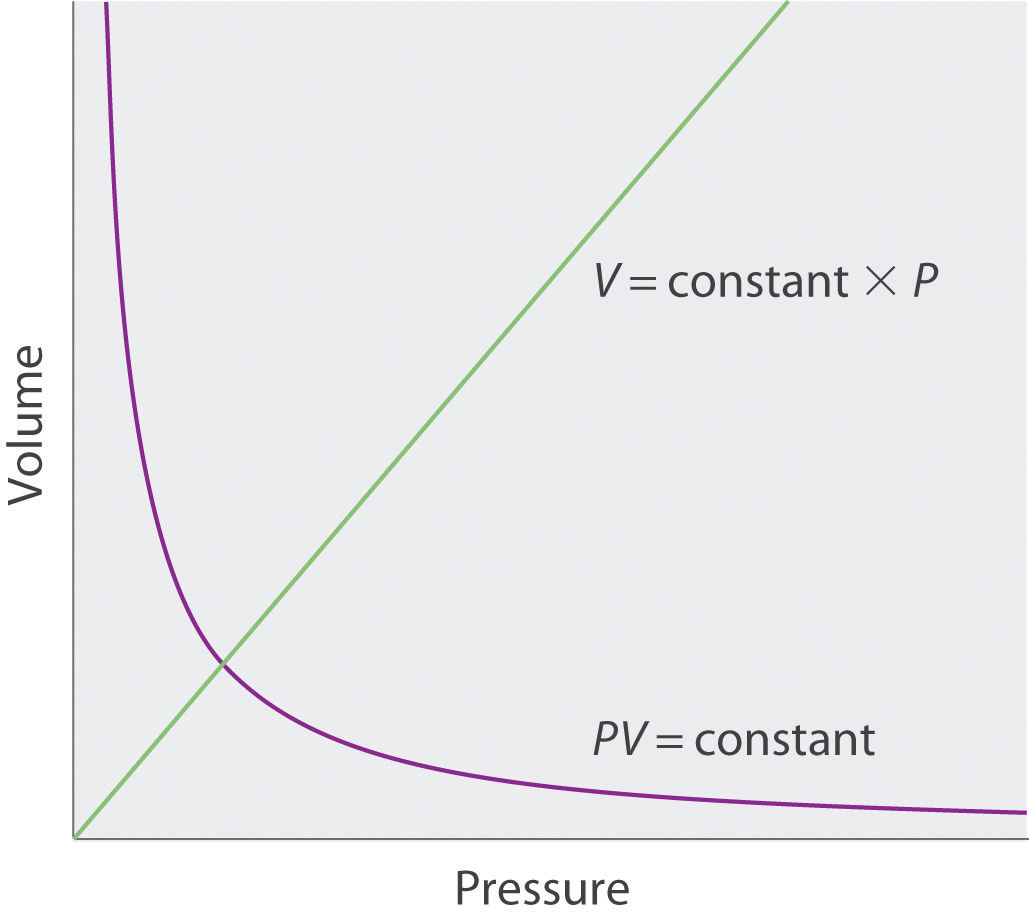 Relationships Among Pressure Temperature Volume And Amount
Relationships Among Pressure Temperature Volume And Amount
 Relating Pressure Volume Amount And Temperature The Ideal Gas
Relating Pressure Volume Amount And Temperature The Ideal Gas

 Is The Density Of The Air In A Heated Hot Air Balloon Less Than The
Is The Density Of The Air In A Heated Hot Air Balloon Less Than The
 Properties Of Matter What Is Compression
Properties Of Matter What Is Compression
 Properties Of Matter What Is Compression
Properties Of Matter What Is Compression
 Multi And Instabilities In Gas Partitioning Between Nanoporous
Multi And Instabilities In Gas Partitioning Between Nanoporous
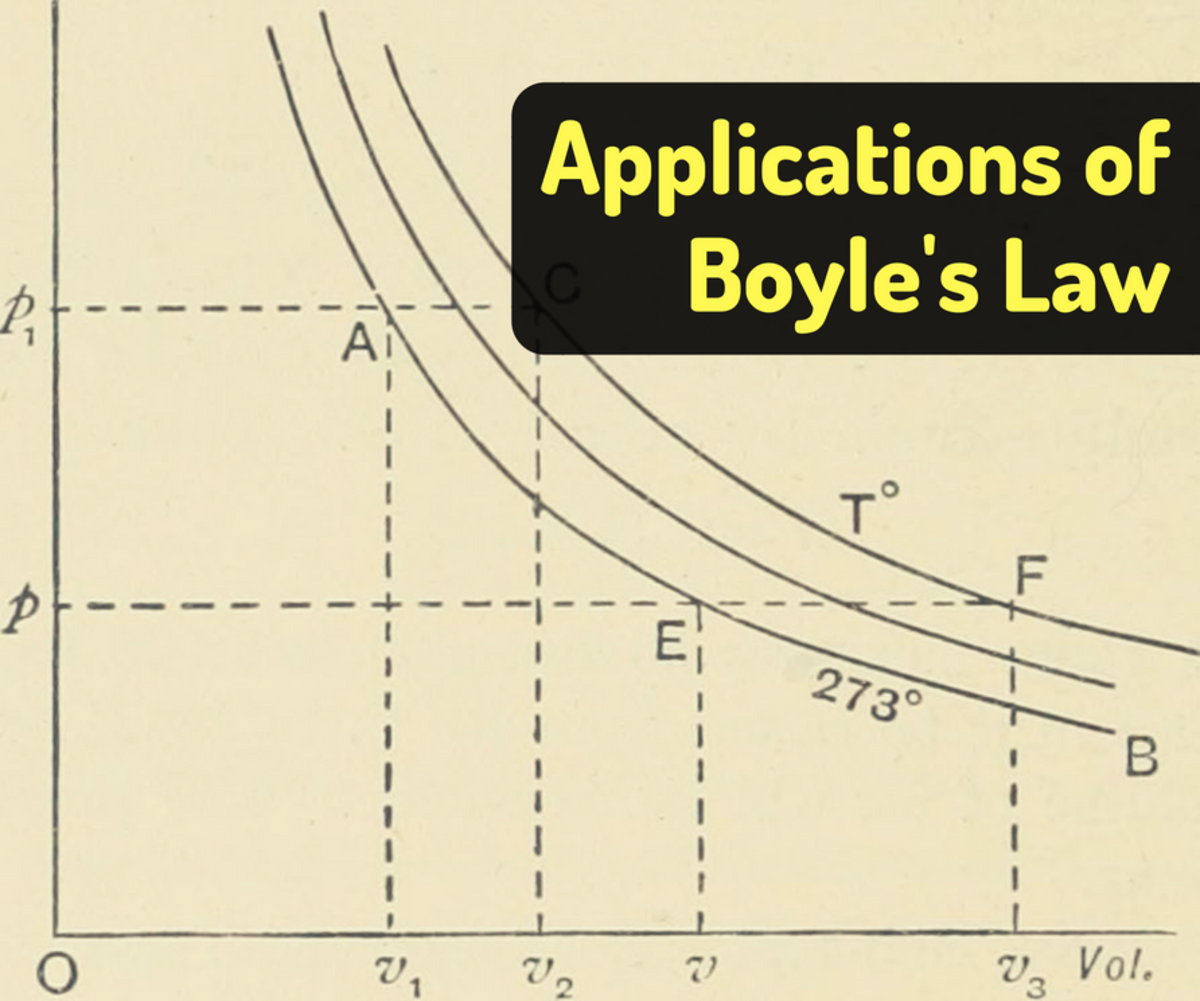 Boyle S Law Examples In Real Life Owlcation
Boyle S Law Examples In Real Life Owlcation
 Gas Laws Why Do Helium Balloons Expand In Volume As They Go Higher
Gas Laws Why Do Helium Balloons Expand In Volume As They Go Higher
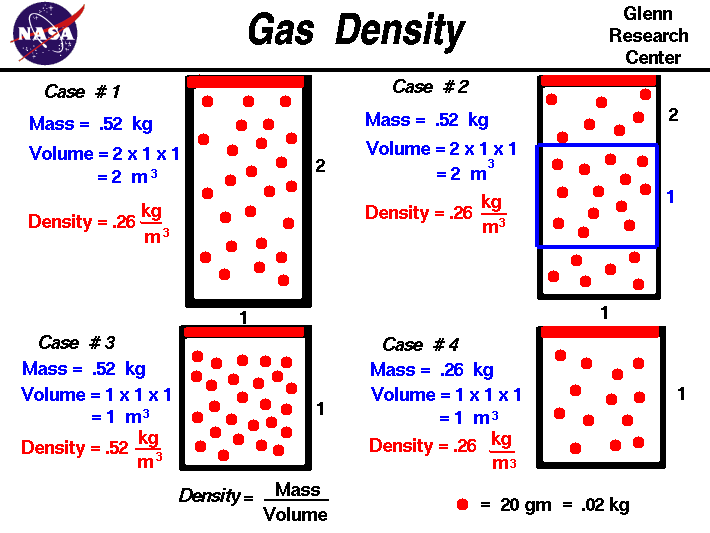

0 Response to "One Way To Increase The Volume Of The Gas In The Balloon In The Diagram Above Is To"
Post a Comment