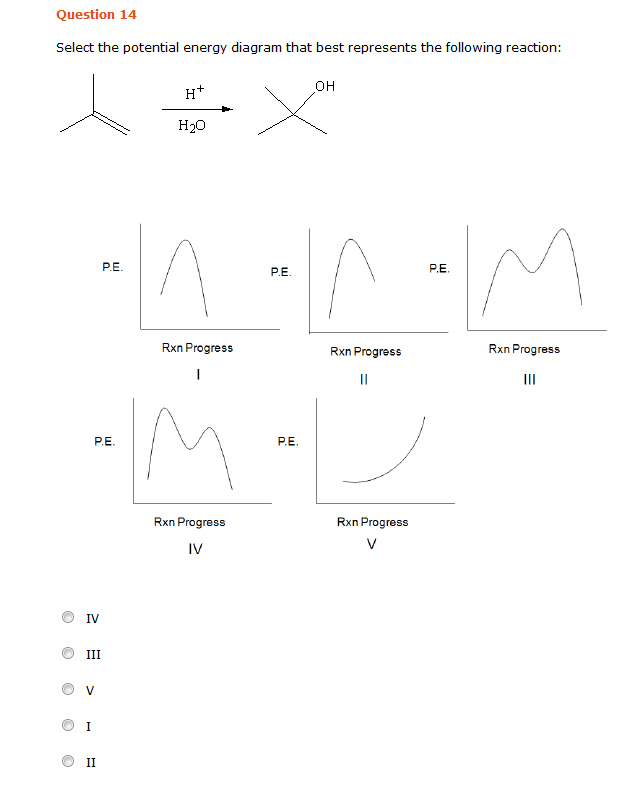
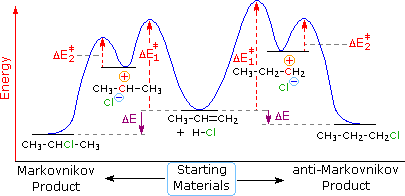
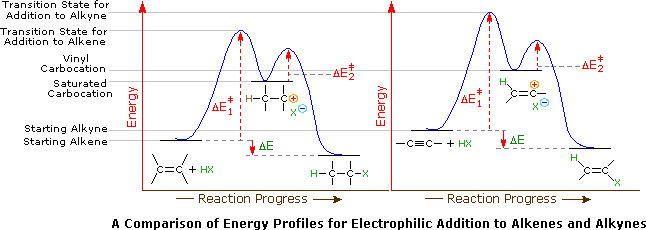
Select The Potential Energy Diagram That Best Represents The Following Reaction
Answer to select the potential energy diagram that represents a two step endothermic endergonic reaction. D subscribe to view the full document.
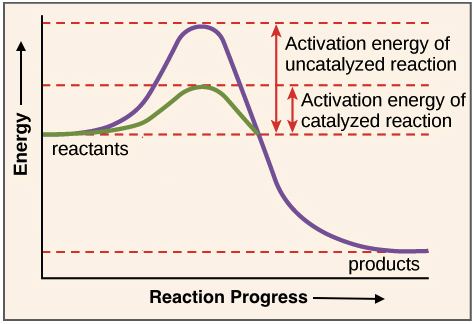
 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
The ice cubes gain heat energy and the water loses heat energy.

Select the potential energy diagram that best represents the following reaction. I hope my answer has come to your help. Select the potential energy diagram that best represents the. Consider the following reaction.
Heat is transferred from the aluminum to the water and the temperature of the water increases. So the enthalpy of reaction is defined as the difference of the energy of the reactants and the energy of the products. Consider the following potential energy diagram the activation energy for the forward reaction is.
Select the potential energy diagram that best represents the following reaction. The required activation energy is greater than the enthalpy change of the reaction based on the potential energy diagram of an endothermic process. The for this reaction will be positive.
This first video takes you through all the basic parts of the pe diagram. H h 2 o oh a i b ii c iii d iv e v ans. A potential energy diagram plots the change in potential energy that occurs during a chemical reaction.
Answer to select the potential energy diagram that best represents the following reaction. What occurs when a 35 gram aluminum cube at 100 degrees c is placed in 90 grams of water at 25 degrees c in an insulated cup. The reactants and products are pretty self explanitory they are the difference between absolute zero and the energy of each on the curve a and g respectively.
A correct conclusion is that the reaction has a. In the given potential energy diagram the energy of product at higher level and energy of reactant at lower level. In the quantum mechanical model the pathway or position of an electron is best represented as irregular electron clouds.
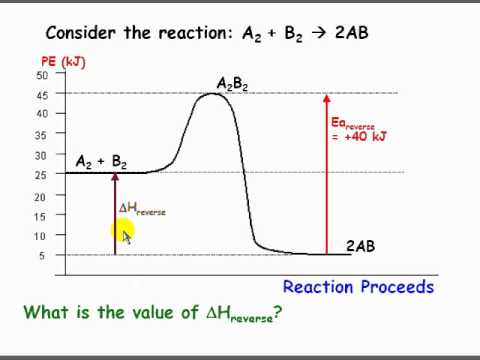
½ h 2g ½ i 2g hi g the activation energy for the formation of hi is 167 kj and for the decomposition of hi is 139 kj. Home study science chemistry chemistry questions and answers select the potential energy diagram that best represents the following reaction. Is negative when heat is released and the reaction is exothermic.
The activation energy is the difference between the reactants and the highest energy state of the reaction the transition point. I ii iii iv. When one mole of a certain compound is formed from its elements under standard conditions it absorbs 85 kj of heat.
The best and most correct answer among the choices provided by your question is the third choice or letter b.
 Solved Question 13 Which Would Be The Best Way To Carry O
Solved Question 13 Which Would Be The Best Way To Carry O
 Reaction Mechanism Britannica Com
Reaction Mechanism Britannica Com
 Enzymes Review Article Enzymes Khan Academy
Enzymes Review Article Enzymes Khan Academy
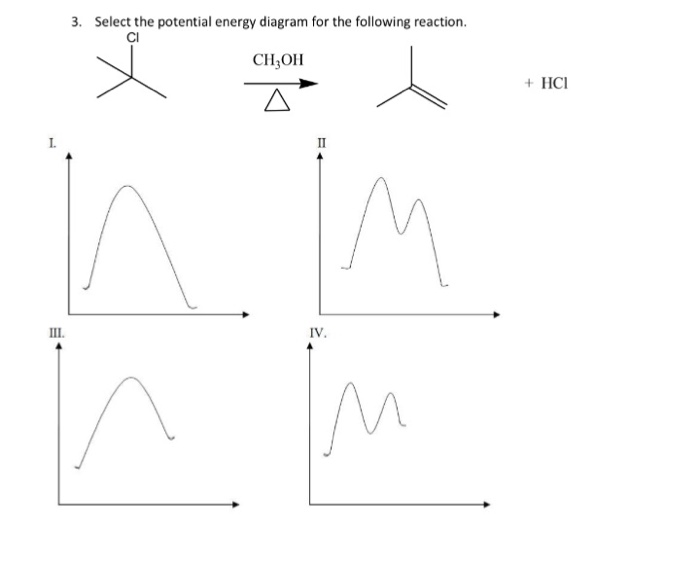
 Select The Potential Energy Diagram That Represents An Exothermic
Select The Potential Energy Diagram That Represents An Exothermic
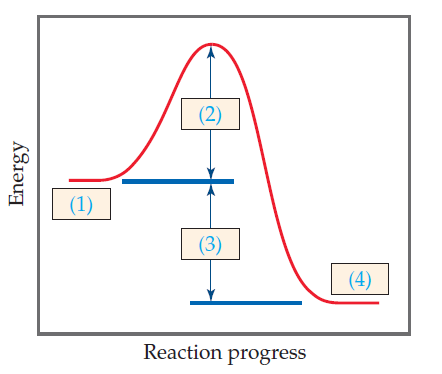 Answer Label The Energy Diagram For A Two Clutch Prep
Answer Label The Energy Diagram For A Two Clutch Prep
 Transition State Theory Chemistry Britannica Com
Transition State Theory Chemistry Britannica Com
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia
 Using Potential Energy Diagrams Flv Youtube
Using Potential Energy Diagrams Flv Youtube
 Energy Profile Chemistry Wikipedia
Energy Profile Chemistry Wikipedia
 Answer Label The Energy Diagram For A Two Clutch Prep
Answer Label The Energy Diagram For A Two Clutch Prep
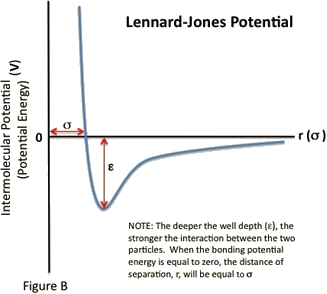 Lennard Jones Potential Chemistry Libretexts
Lennard Jones Potential Chemistry Libretexts
 Ground State Potential Energy Surfaces Around Selected Atoms From
Ground State Potential Energy Surfaces Around Selected Atoms From
 Types Of Energy Knowledge Bank Solar Schools
Types Of Energy Knowledge Bank Solar Schools
 Select The Potential Energy Diagram That Represents An Exothermic
Select The Potential Energy Diagram That Represents An Exothermic
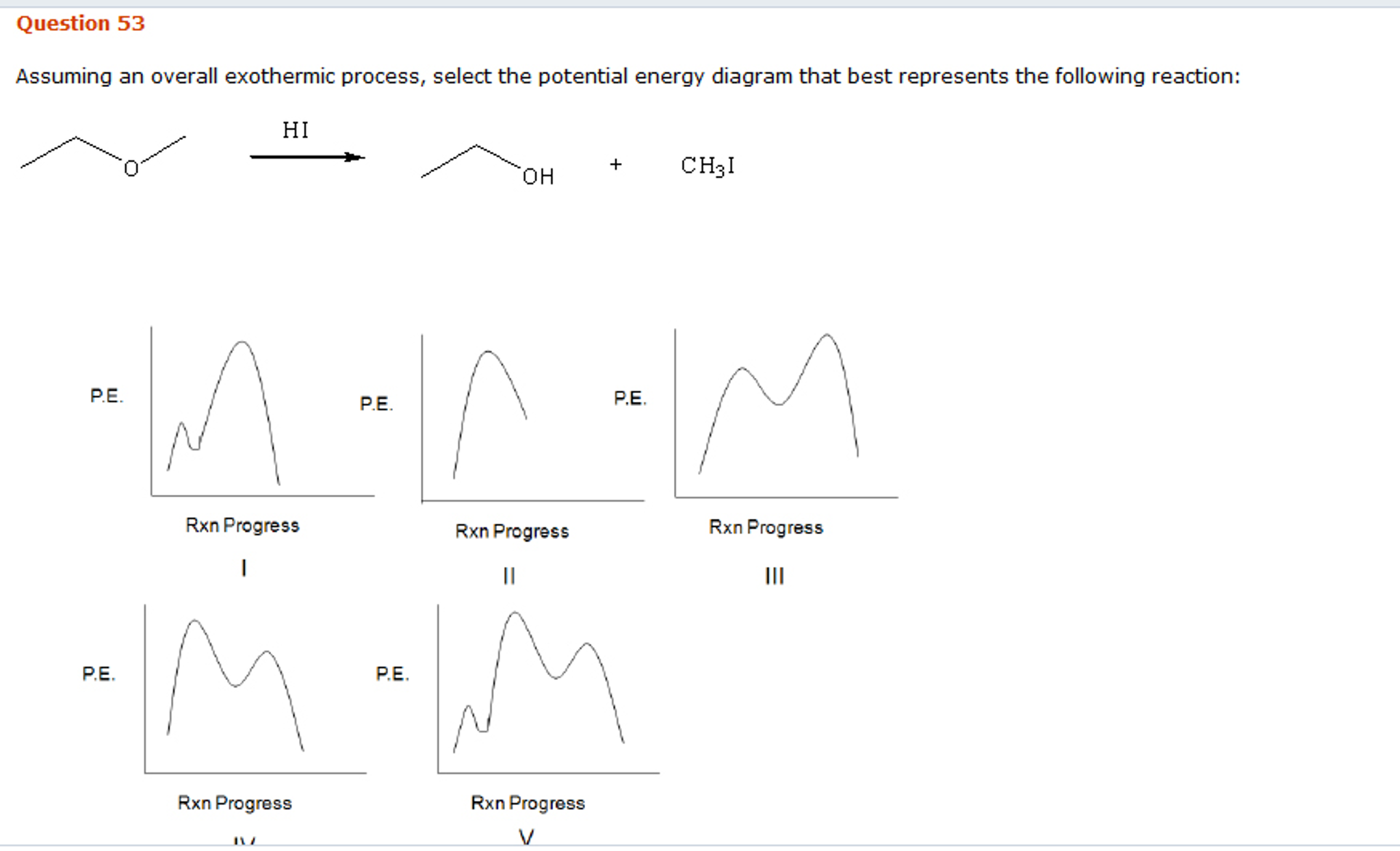 Solved Assuming An Overall Exothermic Process Select The
Solved Assuming An Overall Exothermic Process Select The
 Answer Label The Energy Diagram For A Two Clutch Prep
Answer Label The Energy Diagram For A Two Clutch Prep

 Figure 1 From Probing Potential Energy Surface Exploration
Figure 1 From Probing Potential Energy Surface Exploration
 What Is Chemical Energy Definition Examples Video Lesson
What Is Chemical Energy Definition Examples Video Lesson
Crowdsourcing Enzyme Design With Foldit Conformational Flux
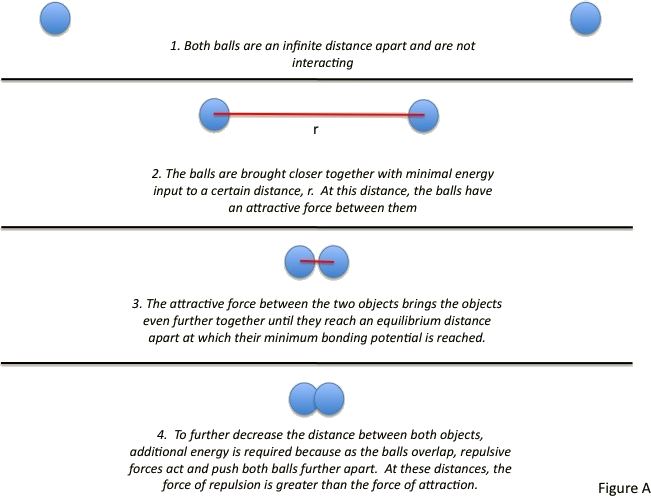 Lennard Jones Potential Chemistry Libretexts
Lennard Jones Potential Chemistry Libretexts

 Activation Energy And The Activated Complex Energy And Chemical
Activation Energy And The Activated Complex Energy And Chemical
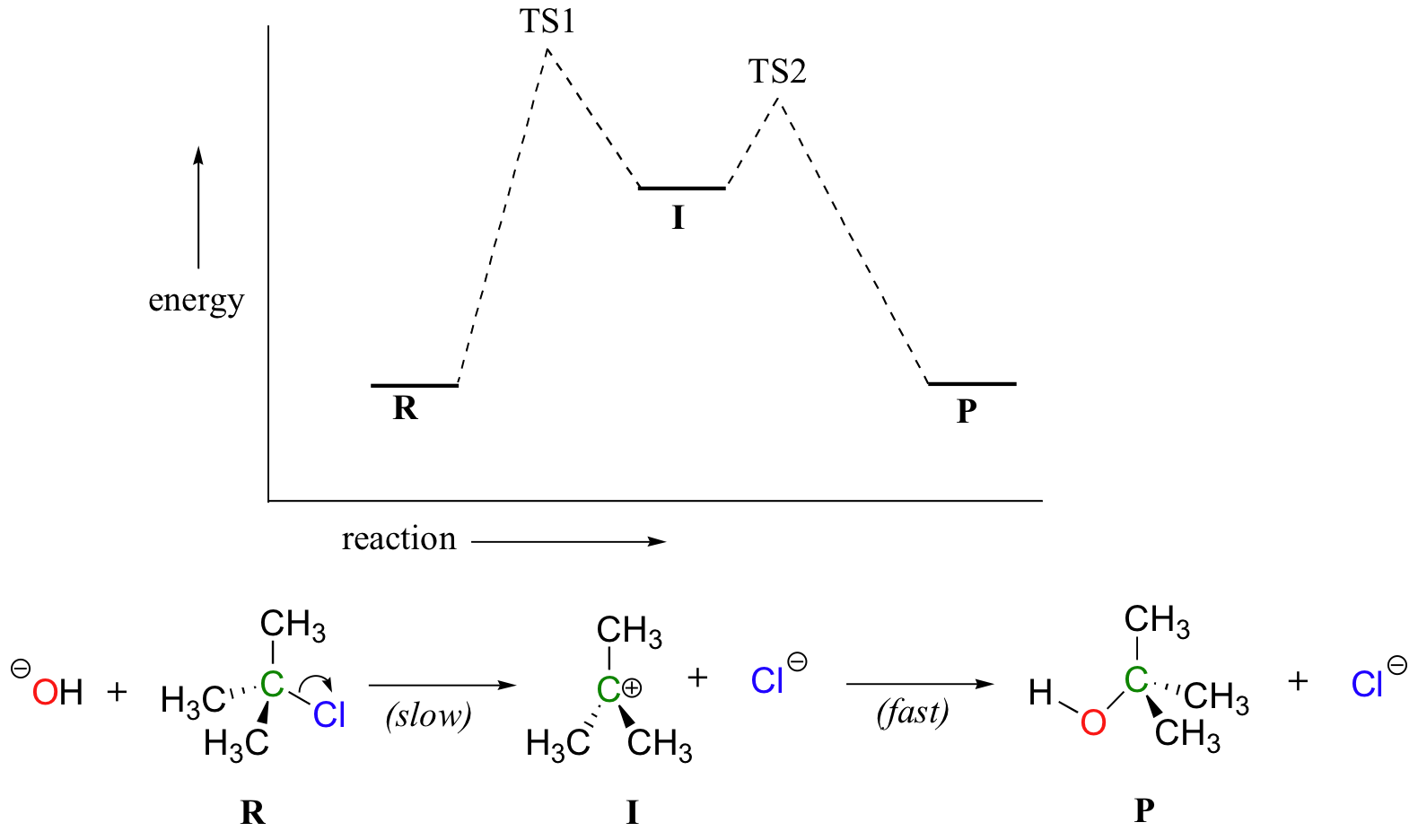 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
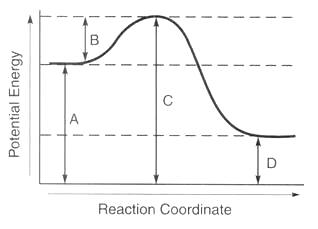
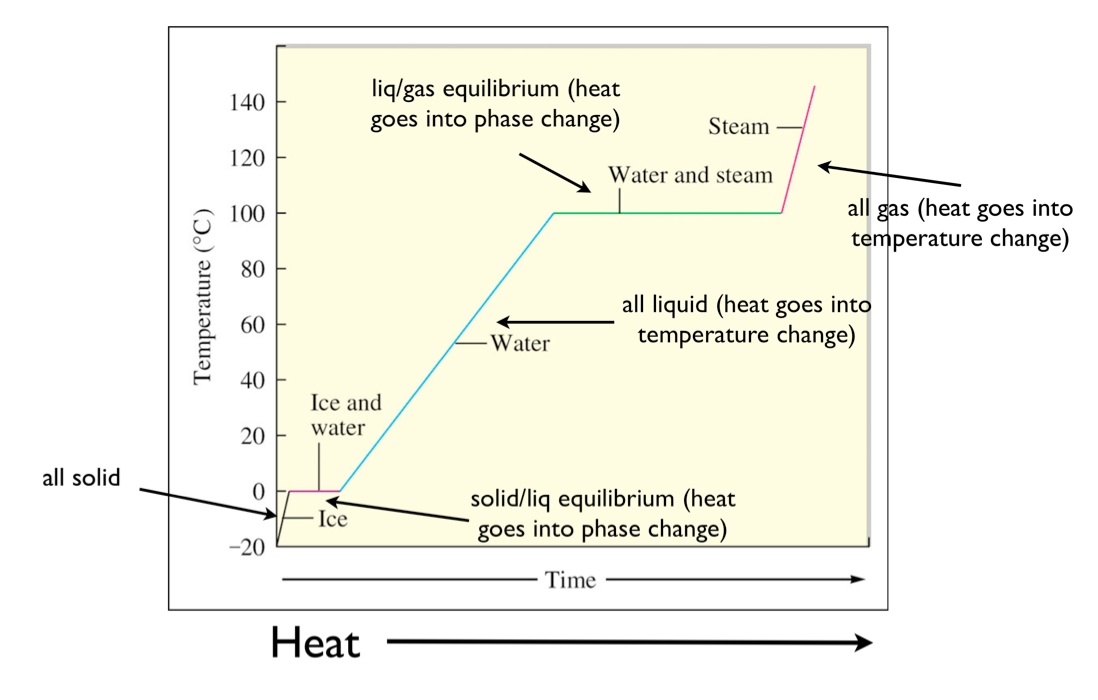




0 Response to "Select The Potential Energy Diagram That Best Represents The Following Reaction"
Post a Comment