Which One Of The Following Is The Correct Orbital Diagram For Nitrogen
A 1s22s22p63s23p63d104s24p6 b 1s22s22p63s23p64s23d104p1 c 1s22s22p63s23p53d104s24p1 d 1s22s22p63s23p64s24d104p1 e none of the above answer b 22 which one of the following is the correct orbital diagram for nitrogen. If you were writing an electron configuration for a iodine atom which elemental symbol would you place in the bracket.
 Solution Given The Molecular Orbital Diag Clutch Prep
Solution Given The Molecular Orbital Diag Clutch Prep
Which one of the following is the correct orbital diagram for nitrogen.

Which one of the following is the correct orbital diagram for nitrogen. Which one of the following is the correct orbital diagram for nitrogen. Answer 19 according to paulis exclusion principle and hunds rule of maximum multiplicity electrons are filled singly in orbitals and pairing starts after all orbitals are singly occupied. Chemistry archive april 27 2016 manicpixi which one of the following is the correct orbital diagram for nitrogen.
O2 calculate the wavelength of the radiation that has an energy of 36 x 10 17 joules. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. There are two shells of electrons in the nitrogen atom that actually have electrons in them nitrogen has two electrons in the first shell the s orbital and five in the outer shell the p.
The remaining three electrons will go in the 2p orbital. 8 protons 8 neutrons and 10 electrons. A specifies the 3 d shape of the orbital.
B specifies the subshell of the orbital. E none of the above. Therefore the n electron configuration will be 1s22s22p3.
Which one of the following is the correct orbital diagram for nitrogen. Use the following information to identify the atom or ion. The cards are meant to be seen as a digital flashcard as they appear double sided or rather hide the answer giving you the opportunity to think about the question at hand and answer it in your head or on a sheet before revealing the correct answer to yourself or studying partner.
D specifies the principal shell of the orbital. An accepted abbreviation format is to write an electron configuration that includes a noble gas symbol in brackets. Flashcards vary depending on the topic questions and age group.
Thus electronic configuration of nitrogen is 1s2 2s2 2p. View the full answer. C specifies the maximum number of electrons.
Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.
Practice Problems Chapter 6 Tst
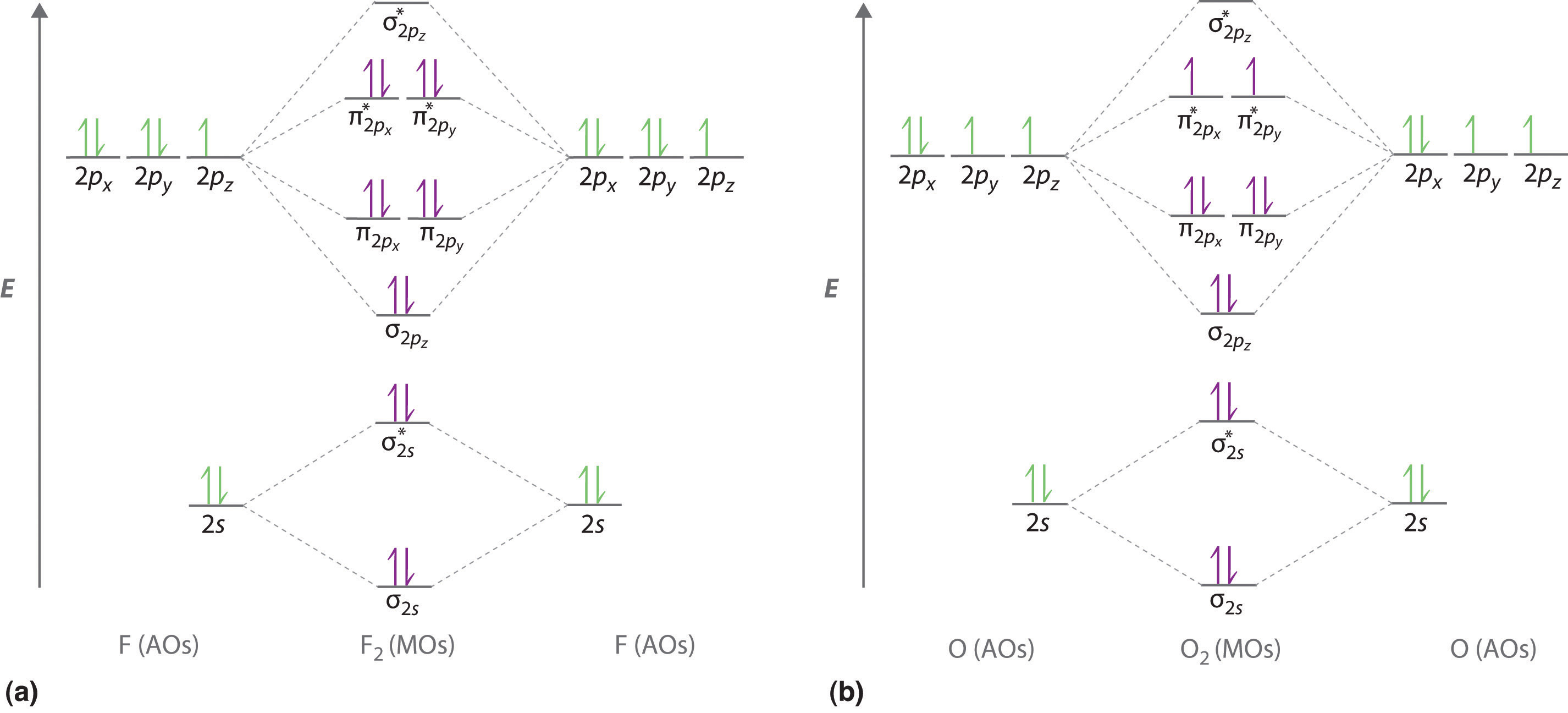 9 8 Second Row Diatomic Molecules Chemistry Libretexts
9 8 Second Row Diatomic Molecules Chemistry Libretexts
 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
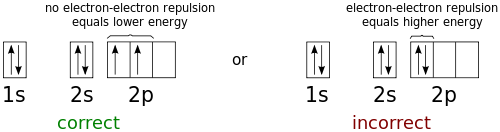 High School Chemistry Orbital Configurations Wikibooks Open Books
High School Chemistry Orbital Configurations Wikibooks Open Books
What Is The Molecular Orbital Diagram For O2 And O2 Ions Quora
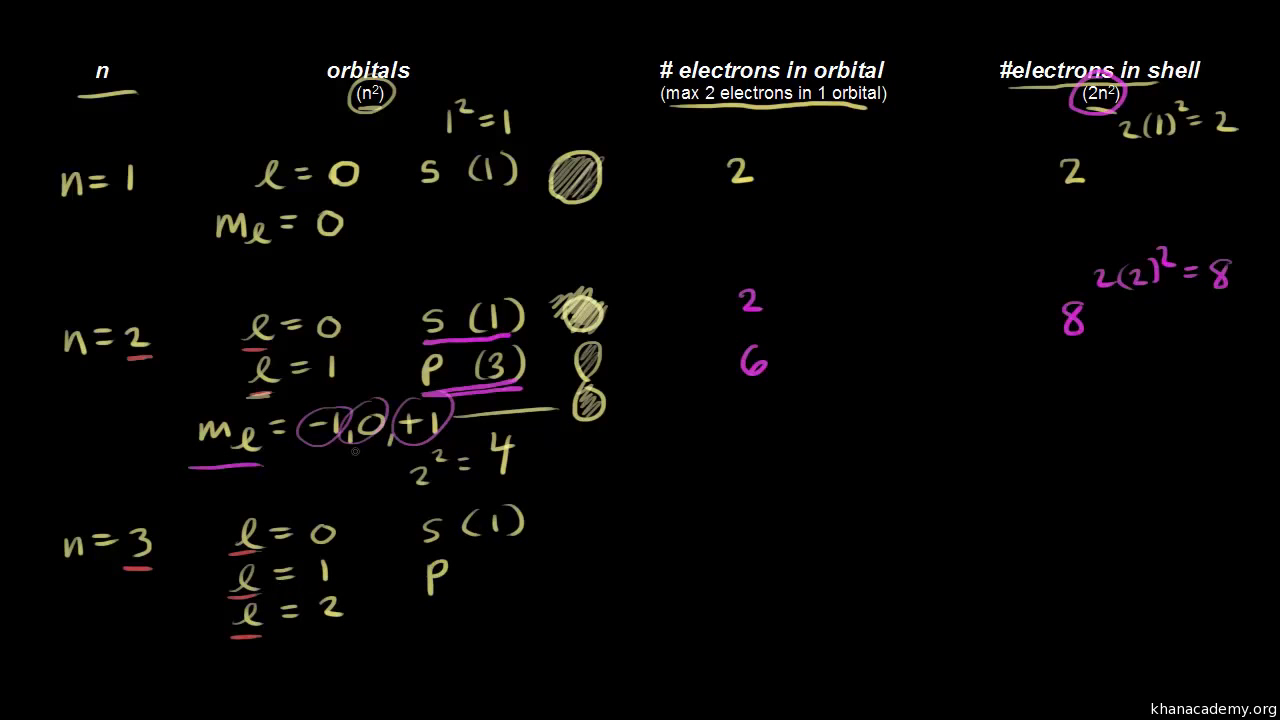 Quantum Numbers For The First Four Shells Video Khan Academy
Quantum Numbers For The First Four Shells Video Khan Academy
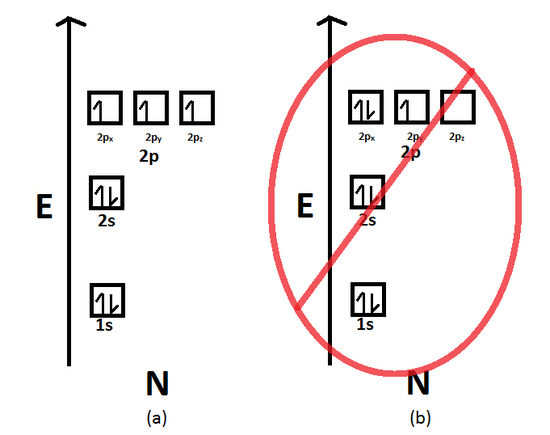 Electron Configuration Chemistry Libretexts
Electron Configuration Chemistry Libretexts
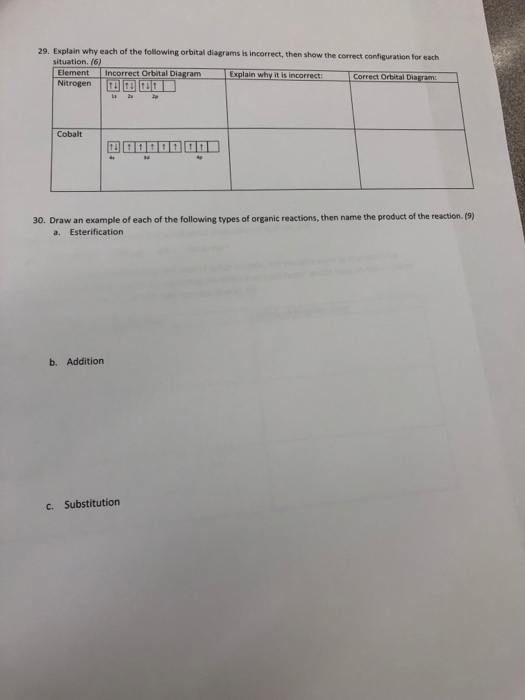
Ch104 Chapter 2 Atoms And The Periodic Table Chemistry
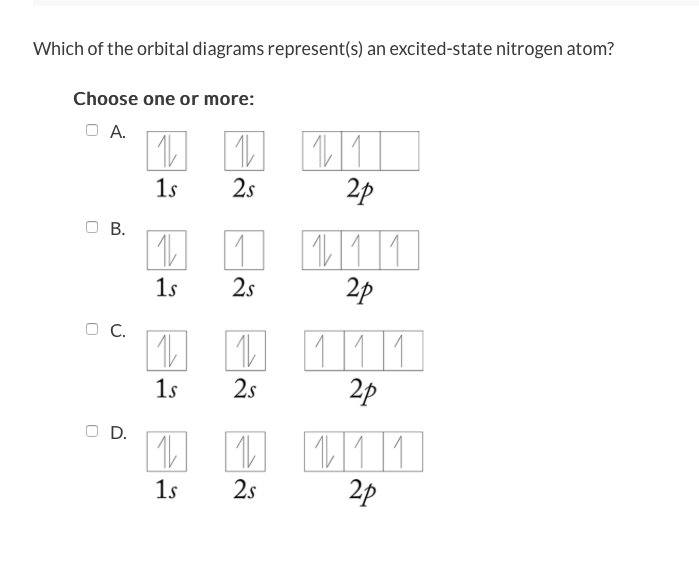 Solved 13 Question 3 Points A See Page 367 Orbital Diagr
Solved 13 Question 3 Points A See Page 367 Orbital Diagr
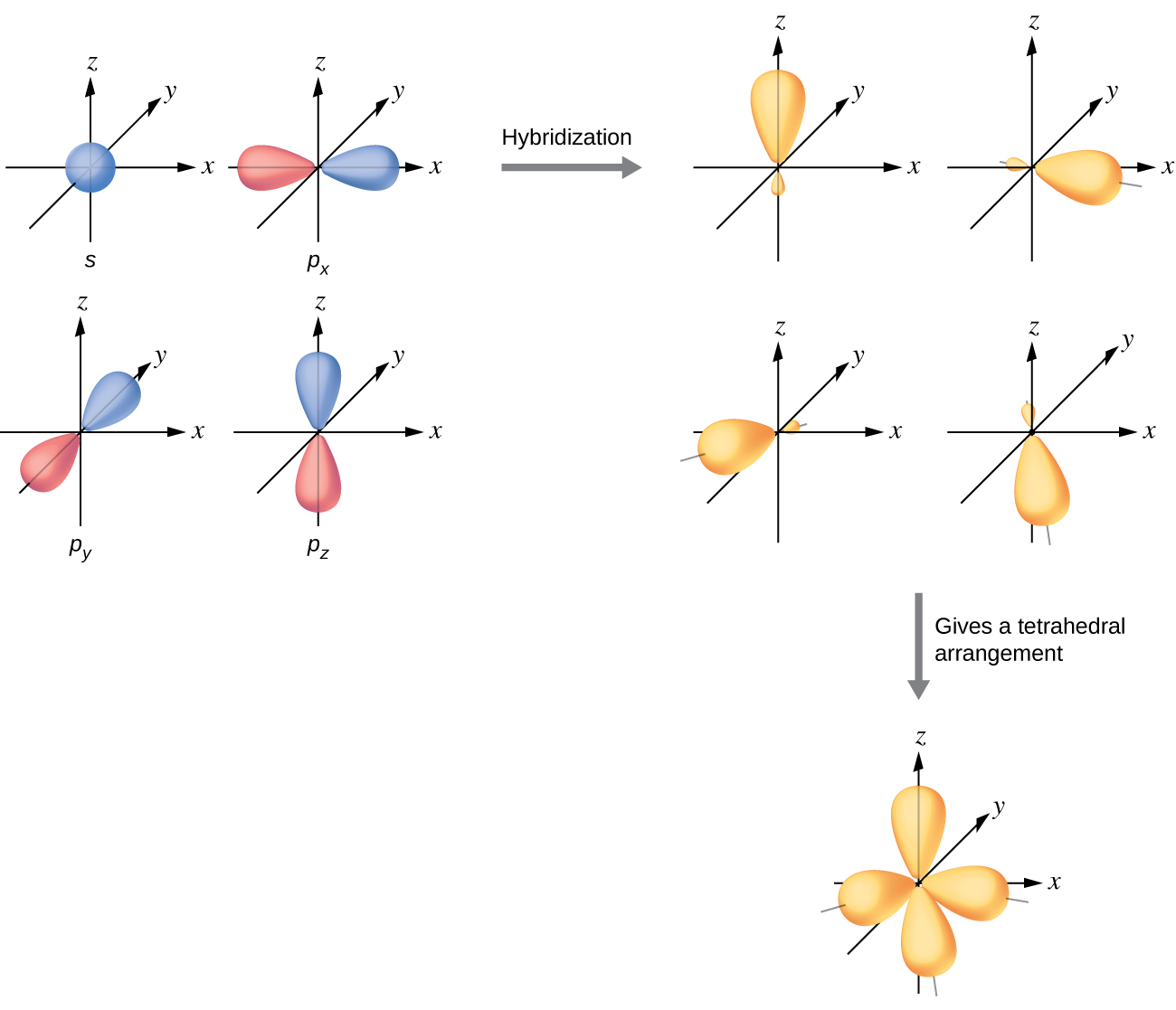 8 2 Hybrid Atomic Orbitals Chemistry
8 2 Hybrid Atomic Orbitals Chemistry

 Using The Mo Diagram Of No Calculate The Bond Order Compare It
Using The Mo Diagram Of No Calculate The Bond Order Compare It
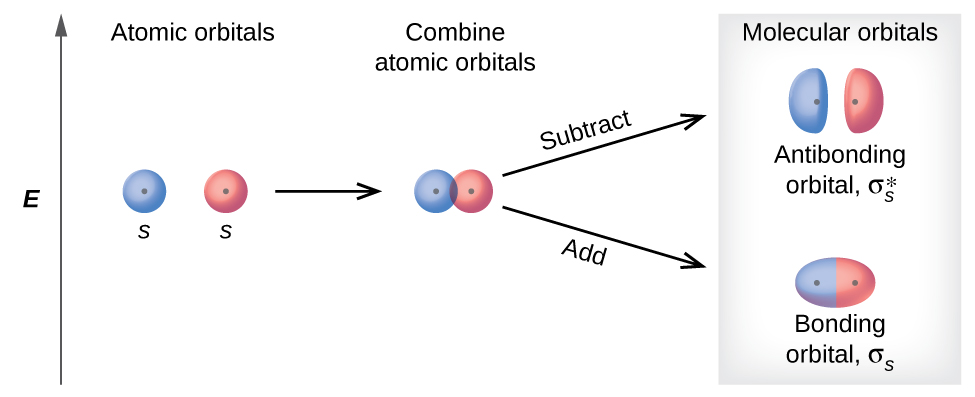 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Hund S Rule And Orbital Filling Diagrams Chemistry For Non Majors
Hund S Rule And Orbital Filling Diagrams Chemistry For Non Majors
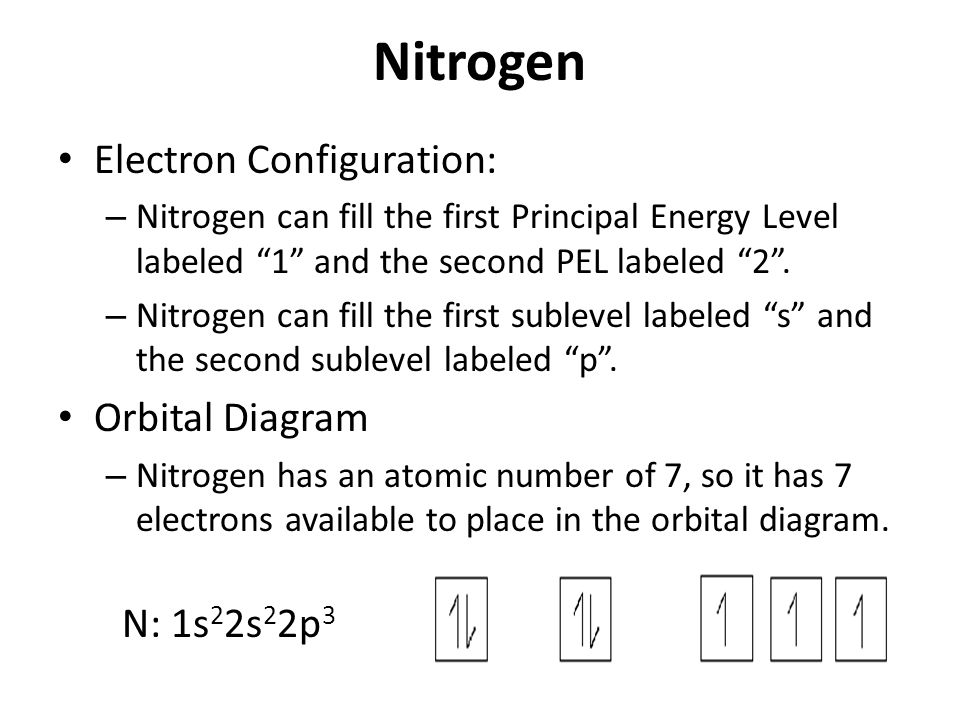 Electron Configuration And Orbital Diagrams Ppt Video Online Download
Electron Configuration And Orbital Diagrams Ppt Video Online Download
 6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
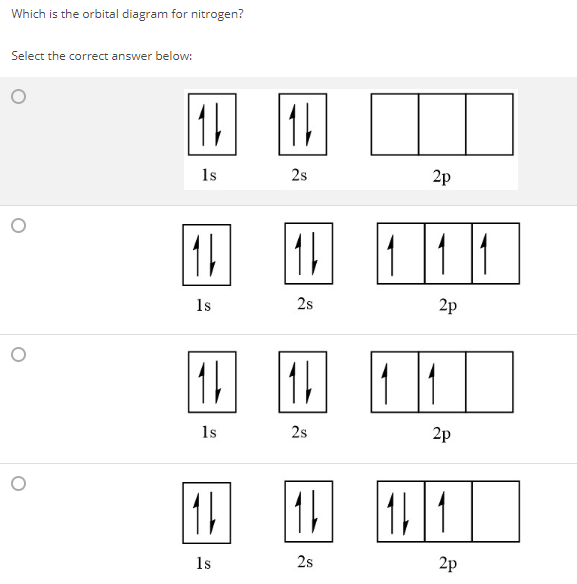 Solved Which Is The Orbital Diagram For Nitrogen Select
Solved Which Is The Orbital Diagram For Nitrogen Select
 Molecular Orbital Energy Level Diagram Of Nitrogen Oxygen Youtube
Molecular Orbital Energy Level Diagram Of Nitrogen Oxygen Youtube
 Frost Diagram Nitrogen Molecular Orbital Diagram Wiring Diagram
Frost Diagram Nitrogen Molecular Orbital Diagram Wiring Diagram
 Final Exam Practice Questions For General Chemistry Pdf
Final Exam Practice Questions For General Chemistry Pdf
Molecular Nitrogen And Related Diatomic Molecules
 Choose The Orbital Diagram That Represents The Ground
Choose The Orbital Diagram That Represents The Ground
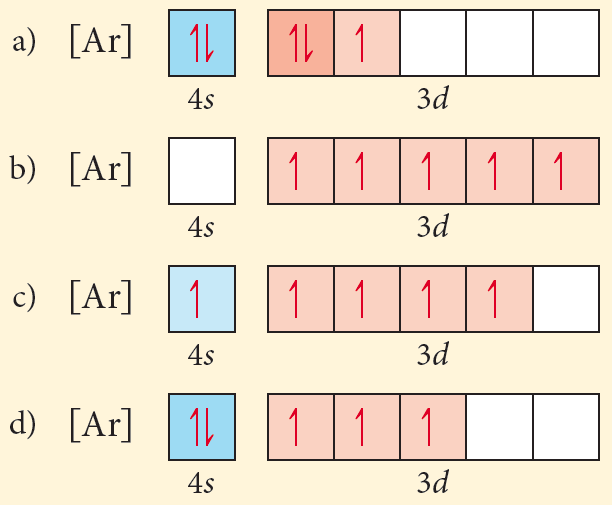 Choose The Orbital Diagram That Represents The Ground
Choose The Orbital Diagram That Represents The Ground
 Inorganic Chemistry Structure Of Nitric Oxide Chemistry Stack
Inorganic Chemistry Structure Of Nitric Oxide Chemistry Stack
0 Response to "Which One Of The Following Is The Correct Orbital Diagram For Nitrogen"
Post a Comment